Solve
Guides
0
Question
SN1 reaction undergoes through a carbocation intermediate as follows:
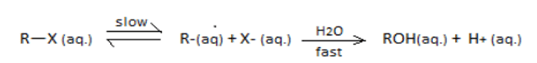
[R = t-Bu, iso-Pr, Et, Me] (X=Cl,Br,I)
The correct statements are:
I. The decreasing order of rate of SN1reaction is t−BuX>iso−PrX>EtX>MeX
II. The decreasing order of ionization energy is MeX>EtX>iso−PrX>t−BuX
III. The decreasing order of energy of activation is t−BuX>iso−PrX>EtX>MeX
Open in App
Solution
Verified by Toppr
I and II are correct.Explanation:(1) Formation of the carbocation is Rate determining step, more stable the cation more easily it will form. Hence rate order is t−BuX>iso−PrX>EtX>MeX(2) Formation of carbocation CH3 is most difficult hence ionization energy is highest. Similarly, EtX has lesser ionization energy than MeX and so on.(3) Order of energy of activation is MeX>EtX>iso−PrX>t−BuX
I and II are correct.
Explanation:
(1) Formation of the carbocation is Rate determining step, more stable the cation more easily it will form. Hence rate order is t−BuX>iso−PrX>EtX>MeX
(2) Formation of carbocation CH3 is most difficult hence ionization energy is highest. Similarly, EtX has lesser ionization energy than MeX and so on.
(3) Order of energy of activation is MeX>EtX>iso−PrX>t−BuX
Was this answer helpful?
0
