Solve
Guides
0
Question
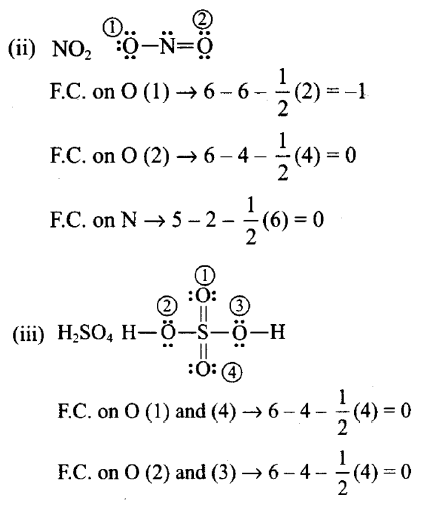
Write lewis structure of the following compounds and show formal charge on each atom $$ HNO_3 , NO_2 ,H_2SO_4$$
Open in App
Solution
Verified by Toppr
Formal charge on an atom in a Lewis structure = [total number of valence electrons in free atom] – [total number of non-bonding (lone pairs) electrons] —1/2 [total number of bonding or shared electrons]

Was this answer helpful?
56