Can you see air? No! Can you eat it? No! Then why do we call it as matter? Well, that is interesting. To understand that, you have to first know what matter is. You would also have to know the various properties of matter. Only then you can truly know why we classify air as matter. Are you ready? Let’s begin the journey through the concept of the matter!
Suggested Videos
What is Matter?
Matter is anything that has mass and occupies some space. So, even if you can’t see air, you can feel it. You know it occupies space. That is why a balloon bursts when you pump extra air into it! Also, you are aware of the fact that air exerts pressure. If it weren’t matter, how would it exert pressure?
Browse more Topics under Some Basic Concepts Of Chemistry
- Atomic Mass and Molecular Mass
- Concentrations
- Dalton’s Atomic Theory
- Importance and Scope of Chemistry
- Laws of Chemical Combination
- Mole and Equivalent Weight
- Nature of Matter
- Percentage Composition
- Stoichiometry and Stoichiometric Calculations
- Uncertainty in Measurement
Classification of Matter
We can classify matter at both micro and macroscopic levels. At the microscopic level, we can classify it into solid, liquid and gas. These are the three physical states of matter. We cannot convert these three states within themselves by applying temperature or pressure.
At the macroscopic level, we can classify matter into pure substances and mixtures. This classification depends on the chemical composition of various substances. Pure substances are further of two types: elements and compounds.
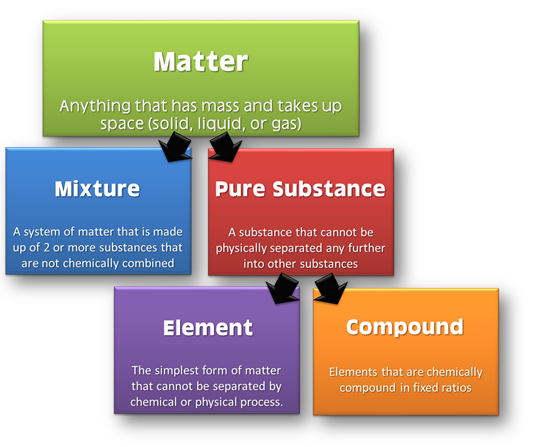
Elements and Compounds
Element refers to the primary stuff present in all the substance. The smaller unit of an element is the atom. Examples of elements include Nitrogen, Hydrogen, Oxygen, Carbon etc. A compound is a pure substance having more than one atom of elements linked by chemical bonds. Chemical reactions result in these bonds. Examples include water.
Mixture
Mixtures refer to the aggregate of more than one type of pure substance. The chemical identity of a mixture remains maintained. Their constituent ratio could vary unlike in compounds. For example, sugar syrup is a mixture of water and sugar.
Physical and Chemical Properties of Matter
Let us look at the physical and chemical properties of matter in a nutshell.
- Physical Properties of matter: These are the properties that we can measure without changing the chemical composition of the substance. The physical properties include mass, volume, density, refractive index etc.
- Chemical Properties of matter: These are the properties that we can evaluate at the cost of matter itself. As an example, we can test the sweet taste of sugar by eating it only.
- Physical Quantities: Many physical properties of matter are quantitative in nature. Such properties of matter are physical properties. The measurement of any physical quantity consists of two parts: the number and the unit. We express the accuracy of the number by using the concepts of significant figures. We express the units of measurement in S.I. units. Usually, we use the concept of dimensional analysis to derive the units.
Units for Measurement
We need to measure all physical quantities. We can express the value of a physical quantity as the product of the numerical value and the unit in which it is expressed.
Fundamental Units
Fundamental units are those units which can neither be derived from one another nor they can be further resolved into any other units. Below, we have listed the seven fundamental units of measurement in S.I. system.
| Quantity | Name of unit | Abbreviation |
| Mass | Kilogram | Kg |
| Length | Meter | m |
| Temperature | Kelvin | K |
| Amount of substance | Mole | Mol |
| Time | Second | S |
| Electric current | Ampere | A |
| Luminous intensity | Candela | Cd |
Derived units
Some quantities are expressed as a function of more than one fundamental units known as derived units. For example velocity, acceleration, work, energy etc.
| Quantity with Symbol | Unit (S.I.) | Symbol |
| Velocity (v) | Metre per sec | ms–1 |
| Area (A) | Square metre | m2 |
| Volume (V) | Cubic metre | m3 |
| Density (r) | Kilogram m–3 | Kg m–3 |
| Energy (E) | Joule (J) | Kg m2s–2 |
| Force (F) | Newton (N) | Kg ms–2 |
| Frequency (n) | Hertz | Cycle per sec |
| Pressure (P) | Pascal (Pa) | Nm–2 |
| Electrical charge | Coulomb (C) | A-s (ampere – second) |
Units and Dimensional Analysis: Conversion of Units
Dimensional Analysis is one of the simplest ways to carry out the calculations involving different units. Here, we convert a quantity expressed in one unit into an equivalent quantity with a different unit. We do this by the use of a conversion factor which expresses the relationship between units.
Original quantity x conversion factor = equivalent quantity
(in former unit) (in other units)
This is based on the fact that ratio of each fundamental quantity in one unit with their equivalent quantity in other unit is equal to one.
Solved Example for You
Q: What is the difference between homogeneous and heterogeneous mixtures?
Ans: A homogeneous mixture has a uniform composition throughout the entire mixture. The common examples include the mixture of sugar or salt in water, iodine in alcohol or any alloy. These have a uniform composition throughout their entirety.
A heterogeneous mixture is one having a non-uniform composition throughout the entire mixture. Examples of such mixtures include the mixture of sodium chloride and iron filings and a mixture of oil and water.






Where are Equivalent weight video in chapter