In today’s day, Polymers are widely used materials, which are found almost in every material used in our routine life. The importance of polymers has become much more emphasized as its applications are numerous and varied. Let us now explore some polymers of commercial importance.
Suggested Videos
Polymers of Commercial Importance
There are various polymers of commercial importance, which are used in so many different dominions of sciences, industry and technology. Some are listed below:
Nylon
Nylon is a synthetic polymer prepared in labs and produced by industries for its commercial importance. It is commonly used in the textile and fabric industry. Nylon comes under the family of linear polyamides.
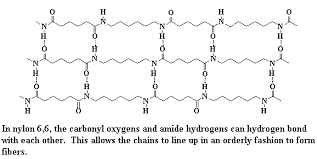
Nylon 6 and nylon 6,6 are 2 commonly used types of nylon. In fact, Nylon 6,6 (widely used as fibres) is made from adipic acid and hexamethylene diamine.They have a compact molecular structure exhibiting excellent abrasion resistance. The monomers are joined by hydrogen bonding. Nylon’s characteristics which make it such a valuable material can be attributed to its strength, lustre, elasticity and resistance to damage by oil and chemicals.
Hydrogen bonding in Nylon 6,6.
Nylon Fiber: Nylon Fibre is used in clothing/apparels in shirts, underwear, raincoats lingeries etc. Industrial uses of Nylon are the production of Conveyer and seat belts, nets and ropes, parachutes and tents.
Browse more Topics under Polymers
- Classification of Polymers
- Types of Polymerisation
- Rubber
- Biodegradable Polymers
- Condensation Polymerisation or Step Growth Polymerisation
Polytetrafluoroethylene
It is commonly known as PTFE is a synthetic fluoropolymer of tetrafluoroethylene. It is of high molecular weight and made up of mainly carbon and fluorine.The best-known brand name of PTFE-based formulas is Teflon which is extensively used in cook-wares such as non-stick pans.
Structure of PTFE
Bakelite
Bakelite is one of the oldest polymers that was synthesized by man. It is a thermosetting polymer and has high strength and retains its shape after moulding. It is one of the first polymer/plastic created in a laboratory. The polymer is formed by condensation of formaldehyde with phenol. Its chemical formula is (C6H6O·CH2O)n.
Bakelite has a high resistance to heat and chemicals and also has a low electrical conductivity due to which it is most commonly used for making electrical switches Bakelite is also used to make the handles of a variety of utensils. It is one of the most important and extensively used polymers for making components and parts of various items. Other uses of this polymer are seen in pipe stems.
Polyvinyl Chloride (PVC)
When talking about polymers of commercial importance we must discuss PVC. It is one of the most widely used polymers in the world. PVC is used extensively across a broad range of applications (used in building, transport, packaging, electrical etc. products), and this can be attributed to its highly versatile nature.
PVC is a highly durable and long-lasting material. It is thermoplastic in nature.It is formed after polymerization of vinyl chloride monomer. It is a very durable and long-lasting material which can be used in a variety of applications, either rigid or flexible, white black or a range of colours in between.
Some common commercial products which use the polymer PV Care window frames, drainage pipe, medical devices, cable and wire insulation. Credit cards and vinyl records are also made using PVC. Recently PVC has also found a place in the textile industry.
Structure of PVC.
Polyethylene Terephthalate
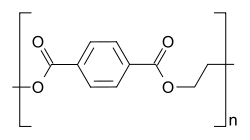
Commonly called PET is a thermoplastic polymer which is extensively used in containers of all kinds. PET consists of polymerized units of the monomer ethylene terephthalate, with its repeating unit as (C10H8O4). PET bottles are commonly recycled, making it very cost effective. This is why it is one of the most important polymers of commercial importance.
Structure of PET
Polypropylene
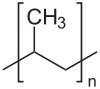
Polypropylene is one of the most versatile polymers produced commercially, with applications both as a plastic and fibre.It is a thermoplastic polymer which is translucent and possesses good chemical and heat resistance. Now, Polypropylene (PP) is a linear hydrocarbon polymer, and its empirical formula is expressed as -(CnH2n)-. The Monomer propylene polymerizes to form polypropylene
Polypropylene is commonly used in consumer products as Furniture, Luggage, Toys, Battery Cases and other “durable” items for the home, garden or leisure use. It is also widely used in packaging ( also reusable containers) and textiles.
Structure of polypropylene
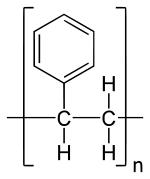
Polystyrene
Polystyrene is a synthetic aromatic polymer which is formed by the polymerization of styrene monomers. And Polystyrene can be solid or foamed. As a hard solid plastic, it is commonly used in rigid a packaging, toys, bottles, lids, refrigerator trays and boxes, disposable cutlery etc.
Foam polystyrene is made in a way where it can have 95 percent air present and is widely used to make home and appliance insulation, surfboards, lightweight protective packaging and food packaging.
Structure of polystyrene
Neoprene
Neoprene, also called polychloroprene or chloroprene rubber is an addition homopolymer, with rubber-like structure and properties. It is a synthetic rubber produced by the polymerization of chloroprene.
Neoprene as a polymer has good chemical stability and is able to maintain flexibility over a wide temperature range. It is used in a wide variety of application, some of the common ones being laptop sleeves, orthopaedic braces and electrical insulation.

(Source: EssentialChemicalIndustry)
Solved Example for You
Q: The catalyst used for the polymerisation of olefins is which of the following?
- Ziegler Natta catalyst
- Wilkinson’s Catalyst
- Pd-catalyst
- None of the above
Sol: The correct answer is option “A”.Ziegler-Natta catalysts are mixtures of titanium compounds like titanium(III) chloride (TiCl3) or Titanium (IV) Chloride (TiCl4) and compounds of Aluminium like Aluminium triethyl Al(C2H5)3. They are used in the synthesis of polymers of 1-alkenes (alpha-olefins). So to polymerize olefins we must use Zeigler Natta catalyst.
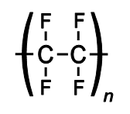








Leave a Reply