Are you aware of the chemical reactions of ethers? We have looked at the chemical properties of ethers, but are they all similar to their chemical reactions? If we ask you about the chemical reactions of ether, would you be able to identify those for us? Well, in this chapter, we will look at the different chemical reactions of ether and understand the behaviour of ethers better.
Suggested Videos
Reactions of Ether
Ethers are relatively unreactive compounds. The ether linkage is quite stable towards bases, oxidizing agents, and reducing agents. Therefore, we must remember that with respect to the ether linkage, ethers undergo just one kind of reaction. It is cleavage by acids :
R-O-R’ + HX → R-X + R’-OH R’ ¾X
Reactivity of HX : HI > HBr > HCl
Cleavage takes place only under quite extreme conditions, like in concentrated acids (usually HI or HBr) and high temperatures. A dialkyl ether produces, initially, an alkyl halide and an alcohol. This alcohol may react further and form a second mole of alkyl halide. For example :

The oxygen of an ether is basic, similar to the oxygen of an alcohol. The initial reaction between an ether and an acid is no doubt, the formation of the protonated ether. Cleavage, then, involves the nucleophilic attack by a halide ion on this protonated ether, with the displacement of the weakly basic alcohol molecule.
Such a reaction usually occurs much more readily as compared to the displacement of the strongly basic alkoxide ion from the neutral ether.

Browse more Topics under Alcohols Phenols And Ethers
- Chemical Reactions of Alcohols, Phenols & Ethers
- Introduction and Classification of Alcohols, Phenols & Ethers
- Nomenclature
- Physical Properties of Alcohols, Phenols and Ethers
- Preparation of Alcohols
- Physical Properties of Ethers
- Preparation of Ethers
- Preparation of Phenols
- Some Commercially Important Alcohols
1) Reactions of Ether Due to an Alkyl Group
- Combustion: Ethers are highly inflammable and they form extremely explosive mixtures with air giving CO2 and water.
C2H5O C2H5 + 6O2 → 4CO2 + 5H2O
- Halogenation: The alkyl group undergoes substitution reaction with chlorine or bromine. The resultant product is halogenated ether in absence of sunlight. However, in presence of sunlight, it substitutes all the hydrogen atoms of ethers.
CH3CH2OCH2CH3 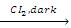 CH3CHCIOCHCICH3 (α α’-dichloro diethyl ether)
CH3CHCIOCHCICH3 (α α’-dichloro diethyl ether)
CH3CH2OCH2CH3 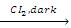 C2CI2OC2CI5 (Perchloro diethyl ether)
C2CI2OC2CI5 (Perchloro diethyl ether)
2) Reaction of Ether Due to Ethereal Oxygen
Ethers behave as Lewis bases because of the presence of two lone pairs of electrons on the oxygen atom. Therefore, they form salts with strong acids. The oxonium salts are soluble in acid solution. We can facilitate the regeneration of ether by hydrolysis of these salts.
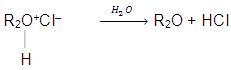
Ethers also form coordination complexes with Lewis acids like BF3, AICI3, RMgX etc. Therefore, we can derive the fact that ethers are very good solvents for Grignard reagents.
3) Formation of Peroxides
Ethers form peroxide linkage with oxygen when we expose them to air or ozonized oxygen in presence of sunlight or ultraviolet light. These peroxides are highly poisonous in nature. They are oily liquids and decompose violently even at low concentrations. Therefore, we must ensure never to evaporate esters to dryness. It might lead to explosive reactions.
Besides this, we must also check the purity of ether before its use as an anaesthetic agent. An impure ether (having peroxide linkage) gives red colour when shaken with ferrous ammonium sulphate and potassium thiocyanate. This could prove to be lethal for the patients on whom we try anaesthesia.

On mixing with KI solution, it liberates I2 which turns starch paper blue.
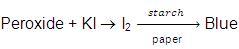
We can make these ethers free from peroxide linkages by distilling them with highly concentrated sulphuric acid, H2SO4. Also, we can check for the peroxide formation by adding a little amount of Cu2O to the ether.
4) Reactions of Ether Involving Cleavage of Carbon-Oxygen Bond
- Action of dil. H2SO4 : Ethers, on heating with dilute H2SO4 , under high pressure, hydrolyse to corresponding alcohols.
- Action of Conc. H2SO4 : Ethers, on warming with conc. H2SO4 , give alkyl hydrogen sulphate.
R-OR + conc. H2SO4 → 2R HSO4
R-OR’ + conc. H2SO4 → RHSO4 + R’HSO4
- Action of HI:
The products that we get during the action of HI on ethers depend mainly upon the temperature in which we carry out the reaction.
R-OR + HI 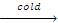 R-OH + RI
R-OH + RI
R-OR’ + HI 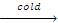 R’-OH + RI
R’-OH + RI
Note: In case of a mixed ether, halogen atom attaches itself to the simpler alkyl group.
CH3OC2H5 + HI → CH3I + C2H5OH
R-R + HI 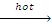 2RI + H2O
2RI + H2O
We would observe similar reactions with HCI, HBr & the reactivity order is HI > HBr > HCI.
- Action of PCI5 : In the presence of heat, we get the following reaction:
R-O-R + PCI5 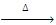 2RCI + POCI3
2RCI + POCI3
- Action of Acetyl chloride or Acetic anhydride :
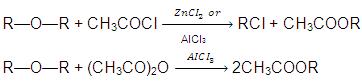
- Dehydration of Ethers:
C2H5OC2H5 ![]() 2CH2=CH2 + H2O
2CH2=CH2 + H2O
- Action of Carbon Monoxide :
C2H5OC2H5, + CO ![]() C2H5COOC2H5
C2H5COOC2H5
ROR + CO → RCOOR
You can download Alcohols, Phenols and Ethers Cheat Sheet by clicking on the download button below

Solved Example for You
Q: Write down a few uses of ethers.
Ans: We commonly use ethers as:
- General anaesthetic agent.
- As a refrigerant. This is because it produces cooling on evaporation.
- A solvent for oils, fats, resins, Grignard reagent etc.
- For providing inert & moisture free medium for reactions e.g. Wurtz reaction.







Who wrote this? Year please