You are probably very well versed with the concept and term ‘alcohol’. It is not just what you see people drinking! It is a very common organic compound that finds large-scale practical applications. However, are you aware of the various methods for the preparation of alcohols? In this chapter, we will look at the various industrial methods of preparation of alcohols.
Suggested Videos
Preparation of Alcohols
There are various methods and ways to prepare alcohols in industries and laboratories. Let’s learn about these one-by-one below.

1) Hydrolysis of Alkyl Halides
This is a nucleophilic substitution reaction. The method is not a very effective one. This is because it has as olefins as by-products. ion.
R-X + KOHaq → R-OH
Browse more Topics under Alcohols Phenols And Ethers
- Chemical Reactions of Alcohols, Phenols & Ethers
- Chemical Reactions of Ethers
- Introduction and Classification of Alcohols, Phenols & Ethers
- Nomenclature
- Physical Properties of Alcohols, Phenols and Ethers
- Physical Properties of Ethers
- Preparation of Ethers
- Preparation of Phenols
- Some Commercially Important Alcohols
2) Oxymercuration and Demercuration of Alkanes
Alkenes react with mercuric acetate in presence of H2O and tetrahydrofuran to give alkyl mercury compounds. This is one of the most common types of methods to prepare alcohols.
3) Preparation of Alcohols from Grignard Reagent
We can obtain the three types of monohydric alcohols (primary, secondary and tertiary alcohols) by using Grignard reagents and carbonyl compounds. The addition of RMgX on carbonyl compounds, along with hydrolysis gives us alcohols. The Grignard reagent is basically an organometallic compound. Let us look at this reaction in greater detail as it is a very important reaction.
When we allow a solution of an alkyl halide in dry ethyl ether, (C2H5)O to stand overturnings of metallic magnesium, we witness a vigorous reaction. We can see that the solution turns cloud and begins to boil. The magnesium metal gradually disappears. The resulting solution is the Grignard reagent.
CH3I + Mg 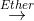 CH3MgI
CH3MgI
H3CH2Br + Mg 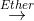 CH3CH2MgBr
CH3CH2MgBr
Ethyl bromide Ethylmagnesium bromide
The Grignard reagent has the general formula R MgX, and the general name alkyl magnesium halide.
More about Grignard Reagent
The Grignard reagent is highly reactive. It reacts with numerous inorganic compounds including water, carbon dioxide, and oxygen, and with most kinds of organic compounds. It is interesting to note that an alkane is such a weak acid that Grignard reagent can displace it by compounds that we might ordinarily consider to be very weak acids themselves, or possibly not acids at all.
Grignard Synthesis of Alcohols
What class of alcohol we obtain from a Grignard synthesis depends upon the type of carbonyl compound that we use in the reaction: formaldehyde, HCHO, yields primary alcohols. On the other hand, aldehydes yield secondary alcohols while ketones, R2CO, yield tertiary alcohols.
How do we get this? It is simple. The number of hydrogens attached to the carbonyl carbon defines the carbonyl compound as formaldehyde, higher aldehyde or ketone. The carbonyl carbon is the one that finally bears the –OH group in the product; here the number of hydrogen defines the alcohol as primary, secondary, or tertiary.
4) Reduction of Carbonyl Compounds
We can also get alcohols by the reduction of aldehydes and ketones. We can reduce aldehydes to primary alcohols and ketones to secondary alcohols. This process can take place by the catalytic hydrogenation or by the use of chemical reducing agents like lithium aluminium hydride, LiAlH4.
Such reduction techniques find an important place in the preparation of certain alcohols that are less available than the corresponding carbonyl compounds. What we must note here is that Sodium borohydride, NaBH4, does not reduce carbon-carbon double bonds, not even those conjugated with carbonyl groups.
5) Reduction of Acids to Alcohols
Lithium aluminium hydride, LiAlH4, is one of the few reagents that can reduce an acid to an alcohol.
4RCOOH + 3LiAlH4 → 4RCH2OH 1oalcohol
Because of the excellent yields it gives, LiAlH4 is a common ingredient in the laboratory for the reduction of not only acids but many other classes of compounds.
6) Other Methods of Preparation of Alcohols
- By the Action of Nitrous Acid on Primary Amines
R-NH2 + HNO2 → R-OH + N2 + H2O
However, under similar conditions, CH3NH2 gives CH3-O-N=O or CH3OCH3
CH3NH2 + 2HNO2 → CH3-O-N=O + 2H2O + N2
OR 2CH3NH2 + 2HNO2 → CH3OCH3 + 2N2 + 3H2O
- By Fermentation
Fermentation is the slow decomposition of complex organic compounds into simpler organic compounds by the activity of enzymes. Enzymes are complex, nitrogenous (proteins), non-living macromolecules of high molecular weight. We usually get these enzymes from living organisms.
This process is usually followed by the evolution of gases like CO2 & CH4. They release a lot of energy and are exothermic in nature. The alcoholic fermentation involves the conversion of sugar into ethyl alcohol by yeast.
You can download Alcohols, Phenols and Ethers Cheat Sheet by clicking on the download button below

Solved Example for You
Q: What are the favourable conditions for fermentation?
Ans: To facilitate fermentation, we must carry out the following:
- Maintain the optimum temperature range for fermentation at 25-30oC. At higher temperatures, the enzymes could coagulate.
- We need to add certain inorganic substances like (NH4)2SO4, or phosphates etc as a food for the ferment cells.
- We must keep the concentration of the fermentation solution very diluted.
- Make sure that the substances like boric acid, mercury slats etc. should not be present as they retard fermentation.
- We must ensure proper aeration during the process of fermentation.






Who wrote this? Year please