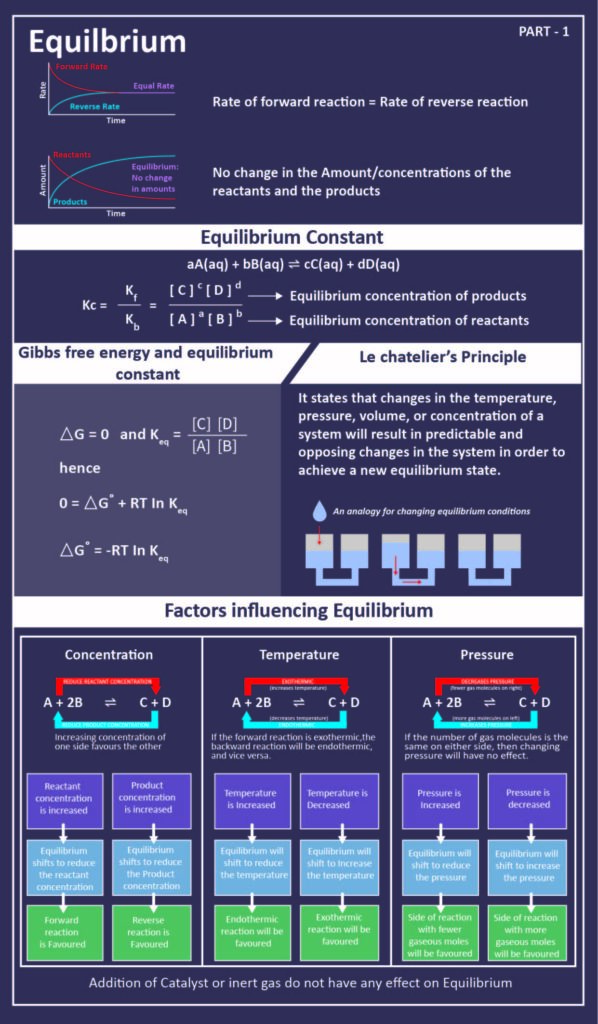
Chemical equilibrium refers to the state wherein both the reactants and the products present in the concentration have no tendency to change with the period of time during a chemical reaction. A chemical reaction achieves chemical equilibrium when the rate of forward reaction and that of the reverse reaction is same. Also, since the rates are equal and there is no net change in the concentrations of the reactants and the products – the state is referred to as a dynamic equilibrium and the rate constant is known as equilibrium constant. Let’s find out more.
Suggested Videos
Law of Chemical Equilibrium
Representation of the attainment of chemical equilibrium is –
The equilibrium constant is defined as the product of the molar concentration of the products which is each raised to the power equal to its stoichiometric coefficients divided by the product of the molar concentrations of the reactants, each raised to the power equal to its stoichiometric coefficients is constant at constant temperature. T
his equilibrium constant can be simply expressed in terms of the partial pressures of the reactants and the products. However, if it is expressed in terms of the partial pressure, it is denoted by Kp.
Browse more Topics under Equilibrium
- Acids, Bases and Salts
- Buffer Solutions
- Equilibrium in Chemical Processes
- Equilibrium in Physical Processes
- Factors Affecting Equilibria
- Ionization of Acids and Bases
- Solubility Equilibria
Equilibrium Constant
Equilibrium Constant Units and Formula
Law of mass action also forms the basis which states that the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants raised to their respective stoichiometric coefficients. Therefore, given the reaction –
aA(g) + bB(g) ⇔ cC(g) + dD(g)
By using the law of mass action here,
- The forward reaction rate would be k+ [A]a[B]b
- The backward reaction rate would be k– [C]c[D]d
where, [A], [B], [C] and [D] being the active masses and k+ and k− are rate constants of forward and backward reactions, also the a, b, c, d are the stoichiometric coefficients related to A, B, C and D respectively. However, at the equilibrium – the forward and the backward rates are equal, stating –
Rate of forward reaction = Rate of backward reaction
![]()
or, 
or, 
where, 
Kc is the equilibrium constant expressed in terms of the molar concentrations. The equation Kc = [ C ]c·[ D ]d / [ A ]a·[ B ]b
or, Kc = Kf / Kb is the Law of Chemical Equilibrium. The equilibrium constant is therefore related to the standard Gibbs free energy change for the reaction which is stated by the equation –
§Gº= -RT ln Keq
where T states the temperature, R is the universal gas constant and Keq is the equilibrium constant.

Solved Examples for You
Question: Write the equilibrium constant expression for the reaction equation:
NH3 + HOAc = NH4+ + OAc–
Solution:
[NH4+] [OAc–] ——————— = K (unitless constant) [NH3] [HOAc]
Question: A closed container has N2O4 and NO2 gases in it. It has been placed in the lab for many days. What would you consider the container and the gases to be?
- an open system
- a closed system
- not a system
Solution: A closed system since it has been in the lab, the temperature of the system is the same as its environment.








Thanks for this. It’s fantastic