As you already know, when physical processes reach a state of equilibrium, it is in a state of physical equilibrium. The same way, when a chemical reaction reaches a state of equilibrium, the reaction is said to be in chemical equilibrium. But what actually happens behind these chemical processes? Let’s find out!
Suggested Videos
Equilibrium in Chemical Processes
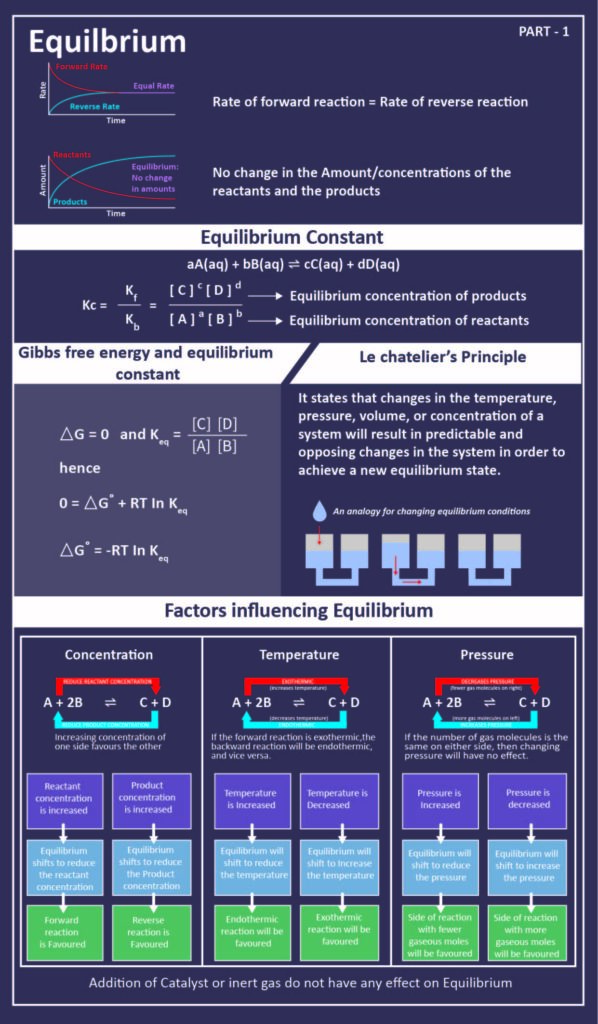
You may have learnt about reversible reactions. As the name suggests, these reactions occur in the forward and reverse direction. In such reactions, when the rate of the forward and the reverse reaction is equal, the concentrations of the reactants and products remain constant. At such a stage, the reaction is said to be in chemical equilibrium.
However, this equilibrium is said to be dynamic in nature. This is because it consists of a forward reaction where the reactants react to give products and reverse reaction where the products can react to give back the original reactants. Let’s understand these chemical processes below.
Browse more Topics under Equilibrium
- Acids, Bases and Salts
- Buffer Solutions
- Equilibrium in Physical Processes
- Factors Affecting Equilibria
- Ionization of Acids and Bases
- Law of Chemical Equilibrium and Equilibrium Constant
- Solubility Equilibria
Dynamic Nature of Chemical Processes in Equilibrium
Let’s consider the following reversible reaction –
A + B ⇌ C + D
As time progresses, the products (C and D) accumulate while the reactants (A and B) deplete. This results in a decrease in the rate of the forward reaction and an increase in the rate of reverse reaction. Ultimately, both reactions occur at the same time reaching a state of equilibrium. This equilibrium can be reached from either direction.
Haber’s Process
The German chemist, Fritz Haber developed a method to manufacture ammonia from dinitrogen and dihydrogen. This process is the Haber’s process.
N2(g) + 3H2(g) ⇌ 2NH3(g)
Haber started out with known amounts of dinitrogen and dihydrogen, maintained at high temperature and pressure, and noted the amount of ammonia produced at regular intervals. As the reaction proceeds, after some time, he noted that the composition of the mixture remains the same even though some reactants are still present. This shows that the reaction has reached equilibrium.

Haber’s process [Source: Wikimedia Commons]
When these two mixtures (H2, N2, NH3 and D2, N2, ND3) were mixed and left for a while, it was observed that the concentration of ammonia was the same as before. Although, mass spectrometry revealed that along with ammonia, all forms containing deuterium and dihydrogen were present (NH2D, NHD2, ND3, H2, HD and D2).
This shows that the scrambling of H and D atoms must be possible due to the continuation of the forward and reverse reactions. There would have been no mixing of isotopes if the reaction had stopped on reaching equilibrium.
Therefore, chemical reactions reach a state of dynamic equilibrium in which the rates of forwarding and reverse reactions are equal and there is no net change in composition.
Chemical Equilibrium is Bidirectional
Whether we start a reaction using reactants or products, equilibrium is attainable from both sides. Let’s consider the following reaction.
H2(g) + I2(g) ⇌ 2HI(g)
If we start the reaction with equal initial concentrations of H2 and I2, then the reaction proceeds in the forward direction where the concentration of H2 and I2 decreases and that of HI increases until it reaches equilibrium.
If we start the above reaction in the reverse direction, then the concentration of HI decreases while that of H2 and I2 increases till it reaches equilibrium. Therefore, if the total number of atoms of an element are same in a given volume, we get the same equilibrium mixture whether we start it with reactants or with products.
Solved Example For You
Question: Haber’s process demonstrates the dynamic nature of chemical equilibrium using which isotope of hydrogen?
- Protium
- Tritium
- Calcium
- Deuterium
Solution: The answer is option D.







Thanks for this. It’s fantastic