Chemical equilibria in a chemical reaction define the state in which there is no further change in the concentration of the reactants and products. In numerous biological and environmental processes, these chemical equilibria play an important role.
One example we can consider for chemical equilibria is that it involves molecules of O2 and the protein haemoglobin play a crucial role in the transportation and delivery of O2 from our lungs to our muscles. Similarly, the equilibria that involve the CO molecules and haemoglobin that accounts and leads to the toxicity of CO.
Suggested Videos
Factors Affecting Equilibria

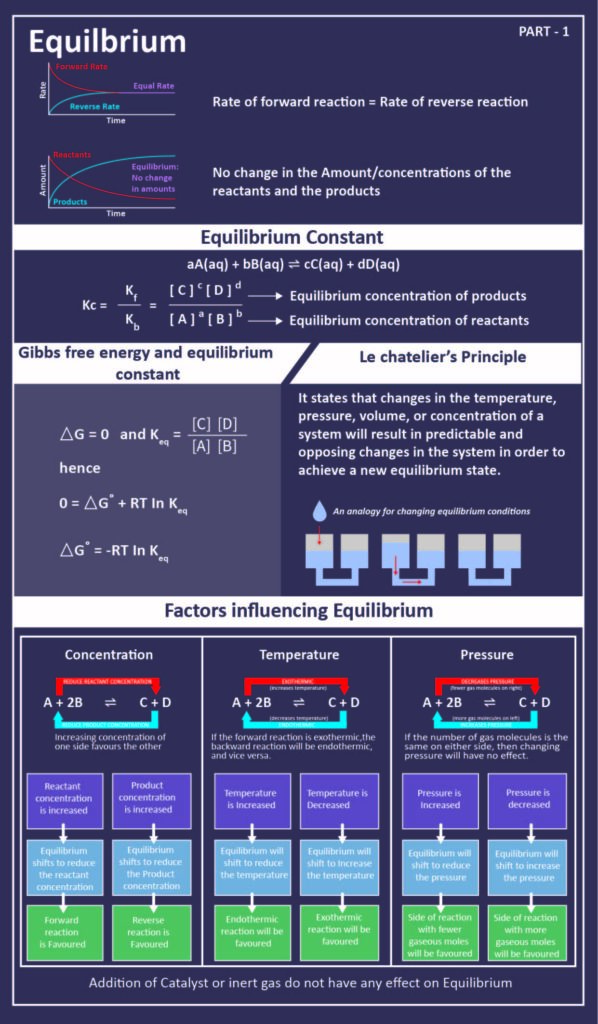
According to the Le Chatelier’s Principle, states that if a system under equilibrium is subjected to a change in pressure, temperature or concentration, in this case, the equilibrium shifts further reducing as well as to counteract the effect of the change. The factors that affect equilibria are:
Effect of Pressure Change
There is no effect of pressure if the number of moles of gaseous reactant and products is equalised. However, it is different for the total number of moles of gaseous reactants and a total number of moles of gaseous products. On increasing the pressure, the total number of moles per unit volume also increases leading to shifting in the equilibrium direction wherein the number of moles per unit volume will be less.
If the total number of moles of products are more than the total number of moles of reactants, in this case, the low pressure will also favour forward reaction. If the number of moles of reactants is more than that of products, high pressure would be favourable to forward reaction.
Effect of Change of Concentration
The equilibrium changes when the concentration of any reactants or products in the reaction changes. It further leads to minimising its effect.
Effect of Inert Gas Addition
After the addition of an inert gas and with the volume kept constant, there is no effect on the equilibrium. This is because, at constant volume, the addition of an inert gas does not change partial pressure or molar concentration.
Effect of Temperature Change
The equilibrium shifts in opposite direction when there is a change of increase or decrease in the system of temperature. This takes effect in order to neutralise the change in effect. In an exothermic reaction, the low temperature favours forwards reaction –
For example. N2(g) + 3H2(G) ——— 2NH3 (g)
∆H = -92.38 kJ mol -1
Low temperature slows down the reaction for which we are using the catalyst. In case of an endothermic reaction, the increase in temperature will shift the equilibrium in direction of the endothermic reaction.
Effect of a Catalyst
There is no effect on the equilibrium composition of a reaction mixture. This is because catalyst increases the speed of both forward and backward reactions to the same extent in a reversible reaction.
Solved Examples for You
Question: In the reaction A + B -> C + D, what will happen to the equilibrium if the concentration of A is increased?
Solution: The reaction will increase in the forward direction.







Thanks for this. It’s fantastic