Why everything tastes so differently? Why lemon is tangy and mango is sweet? This is because of different percentage of acids, bases, and salts in their chemical composition. Let’s learn how these can be characterized, understanding the concepts by Arrhenius, Bronsted-Lowry and Lewis etc.
Suggested Videos
Experimental Definitions
Earlier, acids, bases, and salts were characterized by the experimental testing of their aqueous solutions. An acid is defined as a substance whose water solution tastes sour, turns blue litmus red and neutralizes bases. A substance is called base if its aqueous solution tastes bitter, turns red litmus blue or neutralizes acids.
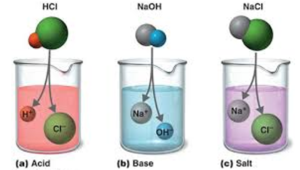
Salt is a neutral substance whose aqueous solution does not affect litmus. According to Faraday: acids, bases, and salts are termed as electrolytes. Further, Liebig proposed that acids are compounds which contain hydrogen that can be replaced by metals.
Acids
Acidity is a characteristic property of acids. Acidic substances are usually very sour. Apart from hydrochloric acid, there are many other types of acids around us. Citrus fruits like lemons and oranges contain citric and ascorbic acids while tamarind paste contains tartaric acid.
In fact, the word ‘acid’ and ‘acidity’ are derived from the Latin word ‘acidus’ which means sour. If you dip a blue litmus paper into an acid, it will turn red while a red litmus paper will not change colour. Acids also liberate dihydrogen when they react with some metals.
Bases
Bases turn red litmus paper blue while the blue litmus paper stays blue. They taste bitter and also feel soapy. Some other common examples of bases include sodium bicarbonate that is used in cooking and household bleach.

Image: Litmus paper test. [Source: Wikimedia Commons]
Salts
Apart from sodium chloride, other common salts are sodium nitrate, barium sulfate etc. Sodium chloride or common salt is a product of the reaction between the hydrochloric acid (acid) and sodium hydroxide (base). Solid sodium chloride is made of a cluster of positively charged sodium ions and negatively charged chloride ions held together by electrostatic forces.
Electrostatic forces between opposite charges are inversely proportional to the dielectric constant of the medium. In other words, we can say that a compound that has acidity in its nature and a compound that has basicity as its nature, may yield salts when combined together.
The universal solvent, water, has a dielectric constant of 80. Therefore, when sodium chloride is dissolved in water, the dielectric constant of water reduces the electrostatic force, allowing the ions to move freely in the solution. They are also well-separated due to hydration with water molecules.

Image: Dissolution of sodium chloride in water [Source: Wikimedia Commons]
Ionization And Dissociation
Dissociation is the separation of ions from an ionic crystal when a solid ionic compound dissolves in water. On the other hand, ionization is the process where a neutral molecule breaks into charged ions when dissolved in a solution. The extent of ionization depends on the strength of the bonds between ions and the extent of solvation of ions.
The three most important modern concepts of acids and bases are:
Arrhenius Concept
According to Arrhenius concept, Substances which produce H+ ions when dissolved in water are called acids while those which ionize in water to produce OH– ions are called bases.
HA → H+ + A– (Acid)
BOH → B+ + OH– (Base)
Arrhenius proposed that acid-base reactions are characterized by acids if they dissociate in aqueous solution to form hydrogen ions (H+) and bases if they form hydroxide (OH–) ions in aqueous solution.

Limitations of Arrhenius Concept
- The presence of water is absolutely necessary for acids and bases. Dry HCl can’t act as an acid. HCl acts as an acid in water only and not any other solvent.
- The concept does not explain the acidic and basic character of substances in non-aqueous solvents.
- The neutralization process is only possible for reactions which can occur in aqueous solutions, although reactions involving salt formation can occur in the absence of a solvent.
- The acidic character of some salts such as AlCl3 in aqueous solution can’t be explained.
- An extended as well as artificial explanation is needed to define the basic nature of NH3.
Bronsted-Lowry Concept
Bronsted and Lowry in 1923 independently proposed a more general definition of acids and bases. According to them, an acid is defined as any hydrogen-containing material (molecule, anion or cation) which can donate a proton to other substance and a Base is any substance(molecule, cation or anion) that can accept a proton from any other substance. Therefore, acids are proton donor whereas bases are proton acceptor.
Conjugate Acid-Base Pairs
Consider a reaction
Acid1 + Base2 → Acid2 + Base1
H2O + HCl ⇔ H3O+ + Cl–
In this reaction, HCl donates a proton to H2O and is, therefore an acid. Water, on the other hand, accepts a proton from HCl, and is, therefore, a base. In the reverse reaction which at equilibrium proceeds at the same rate as the forward reaction, the H3O+ ions donate a proton to Cl– ion, hence H3O+, an ion is an acid. Cl– ion, because it accepts a proton fromH3O+ ion, is a base.
Acid-base pairs in which the members of reaction can be formed from each other by the gain or loss of protons are called conjugate acid-base pairs.
Limitations of Bronsted Lowry Concept
- Bronsted Lowry could not explain the reaction occurring in the non-protonic solvent like COCl3, SO2, N2O4, etc.
- It cannot explain the reactions between acidic oxides like etc and the basic oxides like etc which can easily take place in the absence of solvent as well e.g. (No proton transfer)
- Substances like BF3, AlCl3 etc, do not contain hydrogen which means they can’t donate a proton, still they behave as acids.
Lewis Concept
According to Lewis theory of acid-base reactions, bases donate pairs of electrons and acids accept pairs of electrons. Thus, it can be said that a Lewis acid is electron-pair acceptor.
The advantage of the Lewis theory is that complements the model of oxidation-reduction reactions. Oxidation-reduction reactions take place on a transfer of electrons from one atom to another, with a net change in the oxidation number of one or more atoms.
The Lewis theory further suggested that acids react with bases and share a pair of electrons but there is no change in the oxidation numbers of any atoms. Either an electron is transferred from one atom to another, or the atoms come together to share a pair of electrons.
Al(OH)3 + 3H+ → Al3+ + 3H2O (Aluminium hydroxide is acting as a base)
Al(OH)3 + OH– → Al(OH)4- (Aluminium hydroxide is acting as an acid)
These reactions are showing clearly: When Aluminium hydroxide accepts protons, it acts as a base. When it accepts electrons, it acts as an acid. This Lewis acid-base theory also explains why non-metal oxides such as carbon dioxide dissolve in H2O to form acids, such as carbonic acid H2CO3.
CO2(g) + H2O(l) → H2CO3(aq)
Limitations of Lewis Concept
- Lewis concept gave a generalized idea including all coordination reactions and compounds. This is not true always.
- An idea about the relative strength of acids and bases is not provided by Lewis concept.
- Lewis concept is not in line with the acid-base reaction concept.
- Lewis concept has not discussed the behaviour of protonic acids like HCl.
Solved Example for You
Question: Whether the following ions or molecules can act as Lewis acid or a Lewis base?
- Ag+
- NH3
Solution:
- A silver cation is Lewis acid
- Ammonia is Lewis base









Thanks for this. It’s fantastic