Everyone knows about Hydrogen. It is one of the most abundant elements on earth. It is in the air we breathe, the water we drink, the food we eat. But did you know that atomic hydrogen is not found on Earth? It only exits in its diatomic molecular form as dihydrogen. Let us study learn more about hydrogen.
Suggested Videos
Hydrogen
Hydrogen can be said to be the simplest element in existence. The first element of our periodic table, hydrogen has one electron and one proton. The interesting part is that it does not have any neutrons inside its nucleus! This in turn also makes it the lightest element is known to us.
The discovery of hydrogen was done by Henry Cavendish in 1766. He discovered the inflammable air which was a product of reacting iron with sulphuric acid. The name Hydrogen comes from the Greek word “Hydro” meaning provider since hydrogen produces water on combustion.
Browse more Topics under Hydrogen
- Position of Hydrogen in the Periodic Table
- Hydrides
- Preparation and Properties of Dihydrogen
- Water
- Heavy Water and Hydrogen Economy
- Hydrogen Peroxide
Dihydrogen
A hydrogen atom is extremely reactive in its original form. It has one valence electron in its only shell, which makes it highly reactive and unstable. So hydrogen combines with another atom of hydrogen to give us Dihydrogen. We denote this as H2.
This molecular form of hydrogen is the most common form of hydrogen on earth. Since the molecule is neutral (has no charge) and its orbit is now complete it is a stable gas. Actually, a dihydrogen molecule (H2) is the smallest molecule on the planet.
Isotopes of Hydrogen
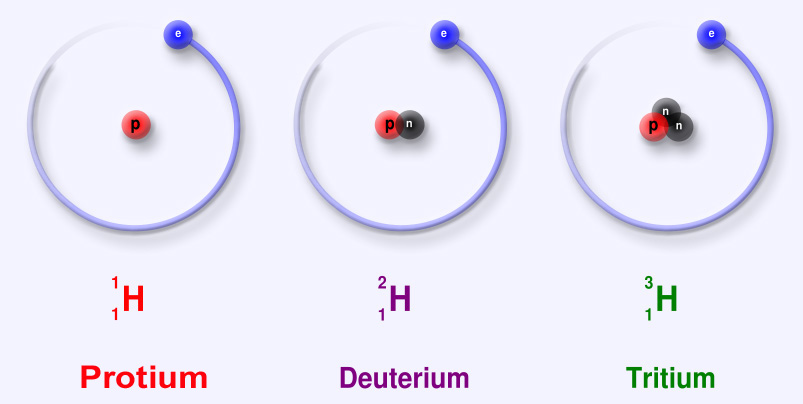
Hydrogen has three naturally occurring isotopes, namely Protium (1H), Deuterium (2H) and Tritium(3H). Of these, tritium is the only one not stable, it is in fact radioactive. The difference between the three is the number of neutrons
- Protium: This is the most prevalent isotope of hydrogen in the world. Over 99% of all hydrogen is in this form. Its name comes from the fact that it has one proton in its nucleus. But it has no neutrons at all. This is why it has a relatively low atomic mass of 1.0078 u.
- Deuterium: Having the atomic symbol 2H or sometimes D. It has one proton and one neutron in its nucleus, giving it the atomic mass of 2.014. It is also known as heavy hydrogen. Most of the deuterium found on earth is found in the oceans or sea water. Deuterium is also a stable isotope, It has the ability to form a water molecule, and such water is known as heavy water.
- Tritium: Tritium is 3H. It has one proton and two neutrons in its structure. It is a radioactive isotope and has a half-life of about 12 years. Tritium also emits low energy β particles.
Solved Example for You
Q: Heavy hydrogen is used for which of the following?
- Filling up balloons
- Studying reaction mechanism
- Oxidizing agent
- All of the above
Sol: The correct option is “B”. We use heavy hydrogen to study reaction mechanism as it is very easy to identify deuterium.
Q: The nuclei of tritium (H3) atom would contain neutrons equal to:
- 1
- 2
- 3
- 4
Sol: The correct option is “B”. The atomic number and mass number of Tritium are 1 and 3 respectively. So it contains 1 electron, 1 proton, and 2 neutrons.






melting point of cacl2 as compared to cal2