How many bonds have you learnt up till now? We are not talking about the relationship bond. Here we are talking about the bonds in chemistry. You must have heard about the covalent bond, ionic bond, etc, right? Now we will see the hydrogen bond in detail in this section. Hearing this for the first time? Or just have a vague idea about it? No worries! We will clear all your doubts. Keep reading below.
Suggested Videos
What is a Hydrogen Bond?
The hydrogen bond is an interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons such as nitrogen, oxygen or fluorine. It is an electrostatic attraction between two polar groups. This occurs when a hydrogen atom (H) is bound to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F), and experiences the electrostatic field of another highly electronegative atom nearby.
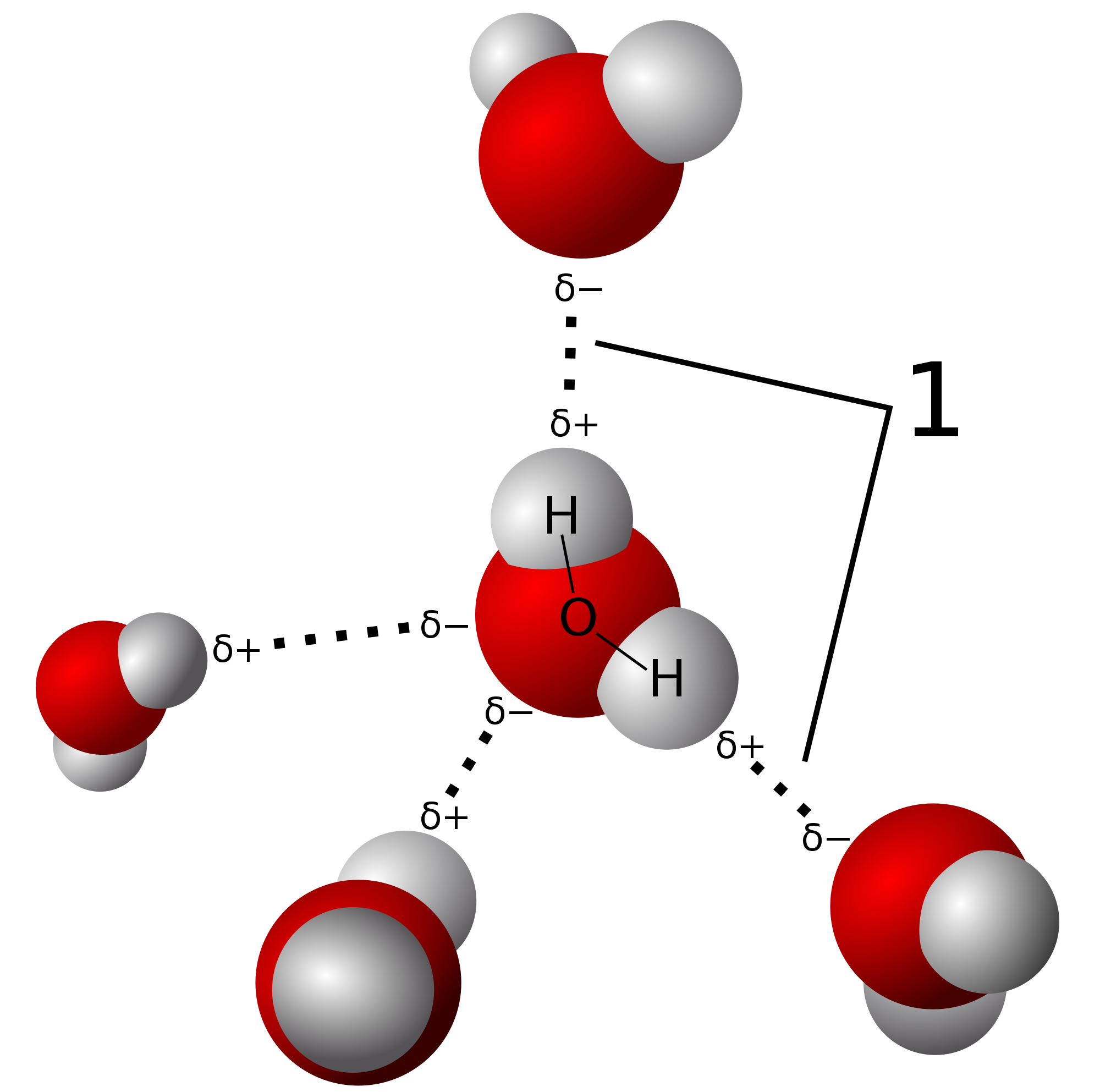
The electrons constituting the covalent bond are shifted toward the more electronegative atom. this happens when there is covalent bond formation between highly electronegative elements and the hydrogen atom. This leads to the development of a partial positive charge on the hydrogen atom which helps in the bond formation with the electronegative atoms of the other molecules. This is hydrogen bond which is comparatively weaker than the covale
Browse more Topics Under Chemical Bonding And Molecular Structure
- Bond Parameters
- Covalent Compounds
- Fundamentals of Chemical Bonding
- Hybridisation
- Hydrogen Bonding
- Ionic or Electrovalent Compounds
- Molecular Orbital Theory
- Polarity of Bonds
- Resonance Structures
- Valence Bond Theory
- VSEPR Theory
Mechanism of Hydrogen Bond Formation
The electrons carry with them a negative charge, so wherever the electrons move they give the negative charge. This results in unequal sharing of electrons. In a molecule, hydrogen bonds are formed, when the hydrogen atom covalently linked to a highly electronegative atom like oxygen, nitrogen, or fluorine, experiences the electrostatic field of another highly electronegative atom of the nearby molecule.
One atom of the pair (the donor), generally a fluorine, nitrogen or oxygen atom, is covalently bonded to a hydrogen atom (-FH, -NH or –OH), whose electrons it shares unequally. Its high electron affinity gives hydrogen a slight positive charge. The other atom of the pair, typically F, N, or O has an unshared pair of the electron; hence it has slight negative charge. Mainly through electrostatic attractions, the donor atom shares its hydrogen with the acceptor atom hence forming a hydrogen bond.
The small sizes of nitrogen, oxygen, and fluorine are essential to hydrogen bonding because it makes those atoms electronegative that their covalently bonded hydrogen is highly positive. Another reason is that it allows the lone pair on the other oxygen, nitrogen, or fluorine to come close to the hydrogen.
Download Chemical Bonding Cheat Sheet PDF by clicking on the Download button below

Hydrogen Bonding in HF Molecule
In HF molecule there is a hydrogen bond between the hydrogen atom of one molecule and the fluorine atom of another molecule.
– – H+– F– – – – H+– F–– – – H+– F–– –
In this case, the hydrogen bond acts as a bridge between two atoms, where one atom is held by a covalent bond and the other atom is held by a hydrogen bond. In the structure above, the dotted line (– – –) depicts the hydrogen bond and the solid line depicts the covalent bond.
The shared pair of electrons move away from the hydrogen atom toward the electronegative atom as the hydrogen atom is bonded to a highly electronegative element. Hydrogen atom becomes electropositive with respect to the electronegative element. This results in the development of positive charge over hydrogen atom and partial negative charge over the electronegative element.
This further leads to the formation of a polar molecule with an electrostatic force of attraction. The magnitude of H-bond depends on the physical state of the compounds. It reaches a maximum value in solid state and minimum in a gaseous state.
- Intermolecular hydrogen bonding occurs between different molecules of same or different compounds. Whereas
- Intramolecular hydrogen bonding occurs when hydrogen atom lies in between the two electronegative elements present in the same molecule.
Hydrogen Bonding in Water
Hydrogen bonds account for some important qualities of water. Even though a hydrogen bond is only 5% as strong as a covalent bond, it’s enough to stabilize water molecules.
- Hydrogen bonding causes water to remain liquid over a wide temperature range.
- As it takes extra energy to break hydrogen bonds, water has an unusually high heat of vaporization. Water has a much higher boiling point than other hydrides.
There are many important consequences of the effects of hydrogen bonding between water molecules:
- Hydrogen bonding makes ice less dense than liquid water, so ice floats on water.
- The effect of hydrogen bonding on the heat of vaporization helps make perspiration an effective means of lowering temperature for animals.
- The effect on heat capacity means water protects against extreme temperature shifts near large water bodies or humid environments. Water helps regulate temperature on a global scale.
Solved Examples for You
Question: On what parameters the energy of hydrogen bond depends?
Answer: The energy of hydrogen atom depends on the nature of donor and acceptor atoms that is their geometry, bond, and environment. The energy can be as high as 40kcal/mol







Leave a Reply