You have now a brief idea of why different elements behave differently. But do you a know a major part of it is because of the “nature” of the bonds in the compounds. Just like you and your best friends have a number of differences due to the “inner” qualities, so is the case with ionic and covalent compounds. In this chapter, we will learn more about the concept of covalent compounds, look at their properties and more.
Suggested Videos
What is a Covalent Compound?
Covalent compounds are the ones having strong intra-molecular bonds. This is because the atoms within the covalent molecules are very tightly held together. Each molecule is indeed quite separate and the force of attraction between the individual molecules in a covalent compound tends to be weak.
We require very little energy in separating the molecules. This is because of the attractive forces between the molecules with the absence of overall electric charge. Covalent compounds are usually gaseous molecules at room temperature and pressure. They might also be liquids with low relatively low boiling points.
These characteristics could be attributed to their weak intermolecular forces which hold these atoms together. However, we also have a lot of solid covalent compounds. They have low melting points. However, it is interesting to note that a small number of these have a completely different structure. They form huge structures where a huge number of atoms are held together. This is possible due to the presence of shared electrons.
These giant molecular structures are basically lattices made up of molecules which are held together by covalent bonds structure. These covalent bonds are very strong. They also tend to be very hard with high melting points which are different from most of the covalent compounds. The example of this kind of covalent compounds includes diamond and graphite of carbon atom network. They also include silica of silicon and oxygen atoms network.
Download Chemical Bonding Cheat Sheet PDF by clicking on the Download button below

Browse more Topics under Chemical Bonding And Molecular Structure
- Bond Parameters
- Fundamentals of Chemical Bonding
- Hybridisation
- Hydrogen Bonding
- Ionic or Electrovalent Compounds
- Molecular Orbital Theory
- Polarity of Bonds
- Resonance Structures
- Valence Bond Theory
- VSEPR Theory
General Properties of Covalent Compounds
- Covalent compounds usually have low melting points. An exception to this include molecules of silica and diamonds that have a high melting point.
- These compounds have low boiling points. This can be attributed to their weak force of attraction between the various bonded atoms. Van Der Waals forces bind these atoms.
- These compounds are usually gases and liquids with low boiling and melting points.
- The solid covalent compounds have soft structures like graphite. This is because of the presence of a cloud of electrons in between each layer of carbons atoms.
- These compounds are non-conductors of electrical charge. The absence of charged ions is the main reason behind this. An exception to this is graphite, where we see a cloud of electrons. These make graphite a good conductor.
- They are bad conductors of heat also. Their molecules lack free electrons and that obstructs the flow of heat energy.
- Covalent compounds do not possess polar characteristics as a general property. Therefore, these compounds are insoluble in water. Water molecules are not absolutely neutral and have a slight negative charge on the oxygen atom and slight positive charges on the hydrogen atoms and since covalent compounds are made up of neutral molecules or molecules with slight charges and hence are not attracted to water molecules strongly.
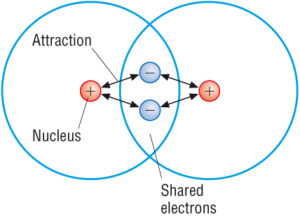
(Source: Google)
Physical And Chemical Properties
- The liquid covalent compounds evaporate. This means the molecules of liquids and solids loses from their surface into the air.
- These compounds have very less affinity between their molecules.
- Various covalent compounds have their own characteristically shaped molecules. Their bonds are directed at pre-set angles.
- Some compounds especially medicines are soluble in water. The rest are soluble in oil.
- Most of the covalent compounds are non-polar or have very little tendency to split completely to form ions and hence never conduct electricity.
- At normal temperature and pressure, we will find these compounds as either liquids or gases. But, there are solids as well and they have higher molecular weights.
- The covalent compounds crystals are of two types: One that has weak van der Waal force holding these together like in Iodine. These are easily fusible and volatile The other having a large network of atoms setting up the macromolecules.
- These compounds are soluble in organic solvents like ether and benzene.
- Covalent bonds are directional in nature. Therefore, they exhibit the phenomenon of isomerism.
- Covalent compounds majorly have a very slow rate of reactions, unlike the various ionic compounds.
Solved Examples for You
Question: Why are covalent compounds not soluble in water?
Answer: Water molecules are not absolutely neutral. These molecules have a slight negative charge on the oxygen atom and slight positive charges on the hydrogen atoms. On the other hand, we know that the covalent compounds are made up of neutral molecules or molecules with slight charges. It is for this reason that these compounds are not attracted to water molecules strongly.







Leave a Reply