In 1896, A.H. Becquerel accidentally discovered radioactivity. He was studying the fluorescence and phosphorescence of compounds irradiated with visible light. This is when he observed something interesting. Let’s learn more about the law of radioactive decay in the section below.
Suggested Videos
He illuminated some pieces of Uranium-Potassium-Sulphate with visible light. Next, he wrapped these pieces in black paper and separated it from a photographic plate by a piece of silver. He left it for several hours. When he developed the photographic plate, he found that there was blackening on the plate.
This meant that something was emitted by the compound which penetrated the silver and black paper and hit the plate. Subsequent experiments show that radioactivity is a nuclear phenomenon which occurs when an unstable nucleus undergoes a decay. This is called Radioactive Decay.
Browse more Topics under Nuclei
- Atomic Mass and Composition of Nucleus
- Mass-Energy and Nuclear Binding Energy
- Nuclear Force
- Nuclear Energy – Nuclear Fusion
- Nuclear Energy – Nuclear Fission
- Radioactivity – Types of Radioactive Decay
Radioactive Decay
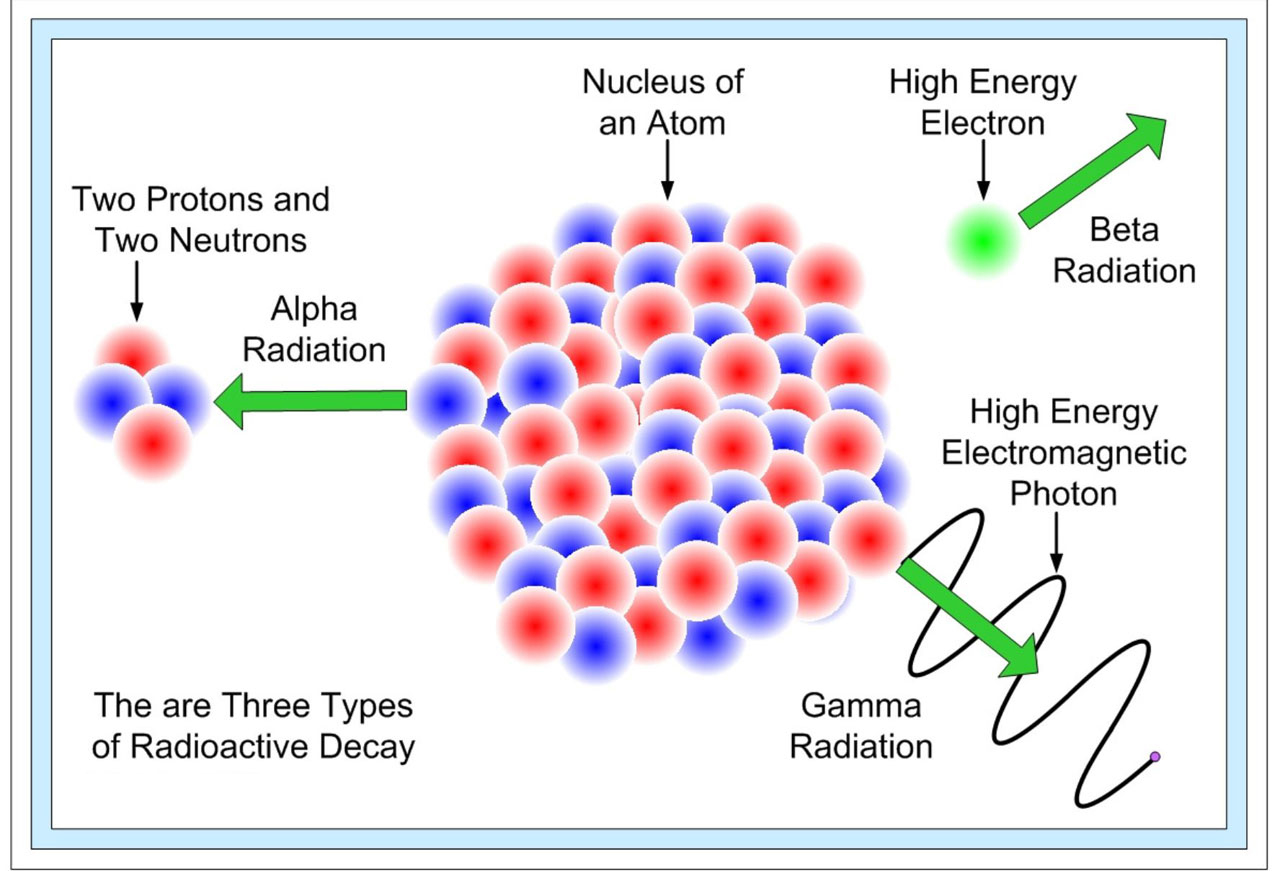
There are three types of radioactive decays in nature:
- α-decay –a helium nucleus (42He) is emitted
- β-decay – where electrons or positrons (particles with the same mass as electrons, but with a charge exactly opposite to that of an electron) are emitted;
- γ-decay – high energy (hundreds of keV or more) photons are emitted.
Law of Radioactive Decay
When a radioactive material undergoes α, β or γ-decay, the number of nuclei undergoing the decay, per unit time, is proportional to the total number of nuclei in the sample material. So,
If N = total number of nuclei in the sample and ΔN = number of nuclei that undergo decay in time Δt then,
ΔN/ Δt ∝ N
Or, ΔN/ Δt = λN … (1)
where λ = radioactive decay constant or disintegration constant. Now, the change in the number of nuclei in the sample is, dN = – ΔN in time Δt. Hence, the rate of change of N (in the limit Δt → 0) is,
dN/dt = – λN
Or, dN/N = – λ dt
Now, integrating both the sides of the above equation, we get,
NN0∫ dN/N = λ tt0∫ dt … (2)
Or, ln N – ln N0 = – λ (t – t0) … (3)
Where, N0 is the number of radioactive nuclei in the sample at some arbitrary time t0 and N is the number of radioactive nuclei at any subsequent time t. Next, we set t0 = 0 and rearrange the above equation (3) to get,
ln (N/N0) = – λt
Or, N(t) = N0e– λt … (4)
Equation (4) is the Law of Radioactive Decay.
The Decay Rate
In radioactivity calculations, we are more interested in the decay rate R ( = – dN/dt) than in N itself. This rate gives us the number of nuclei decaying per unit time. Even if we don’t know the number of nuclei in the sample, by simply measuring the number of emissions of α, β or γ particles in 10 or 20 seconds, we can calculate the decay rate. Let’s say that we consider a time interval dt and get a decay count ΔN (= –dN). The decay rate is now defined as,
R = – dN/dt
Differentiating equation (4) on both sides, we get,
R = λ N0 e−λt
Or, R = R0e−λt … (5)
Where, R0 is the radioactive decay rate at the time t = 0, and R is the rate at any subsequent time t. Equation (5) is the alternative form of the Law of Radioactive Decay. Now we can rewrite equation (1) as follows,
R = λN … (6)
where R and the number of radioactive nuclei that have not yet undergone decay must be evaluated at the same instant.
Half-Life and Mean Life
The total decay rate of a sample is also known as the activity of the sample. The SI unit for measurement of activity is ‘becquerel’ and is defined as,
1 becquerel = 1 Bq = 1 decay per second
An older unit, the curie, is still in common use:
1 curie = 1 Ci = 3.7 × 1010 Bq (decays per second)
There are two ways to measure the time for which a radionuclide can last.
- Half-life T1/2 – the time at which both R and N are reduced to half of their initial values
- Mean life τ – the time at which both R and N have been reduced to, e-1 of their initial values.
Calculating Half-Life
Let’s find the relation between T1/2 and the disintegration constant λ. For this, let’s input the following values in equation (5),
R = (1/2)R0 and t = T1/2
So, we get T1/2 = (ln2)/ λ
Or, T1/2 = 0.693/ λ … (7)
Calculating Mean life
Next, let’s find the relation between the mean life τ and the disintegration constant λ. For this, let’s consider equation (5),
- The number of nuclei which decay in the time interval: ‘t’ to ‘t + Δt’ is: R(t)Δt = (λN0e–λt Δt).
- Each of them has lived for time ‘t’.
- Hence, the total life of all these nuclei is tλN0e–λt Δt
Hence, to obtain the mean life, we integrate this expression over all the times from 0 to ∞ and divide by the total number of nuclei at t = 0 (which is N0).
τ = (λN0 0∞∫ te–λtdt)/N0
= λ0∞∫ te–λtdt
On solving this integral, we get
τ = 1/λ
Therefore, we can summarise the observations as follows:
T1/2 = (ln2)/λ = τ ln 2 … (8)
Solved Examples for You
Question: The half-life of 23892U undergoing α-decay is 4.5 × 109 years. What is the activity of 1g sample of 23892U ?
Answer: T1/2 = 4.5 x 109 years = 4.5 x 109years x 3.6 x 107 seconds/year = 1.42 x 1017 seconds
We know that 1 k mol of any isotope contains Avogadro’s number of atoms. Hence, 1g of 23892U contains, {1/(238 x 10-3)} x 6.025 x 1026 = 25.3 x 1020 atoms. Therefore, the decay rate R is,
R = λN
= (0.693/T1/2)N
= (0.693 x 25.3 x 1020) / (1.42 x 1017)
Therefore, R = 1.23 x 104 Bq






Leave a Reply