Did you know that heat could be transferred between two bodies at different temperatures? In this discussion, we will look into the thermal properties of matter and will discuss various methods of Heat Transfer. We will also learn how to solve problems based on this concept.
Suggested Videos
What is Matter?
We know that matter is defined as anything which has a mass and occupies some space. Matter exists in 4 forms which are solid, liquid, gas and plasma.
What is Heat?
Heat is a form of energy. It derives its origins at the molecular scale. Molecules of a substance vibrate at their positions either fixed or not when energy is supplied to them. As they vibrate they transfer their energy to the surrounding molecules causing them to vibrate as well.
This kinetic energy builds up on a macro level as more and more energy is supplied to these molecules of the substance. As a result, when this energy reaches a threshold ( eg. Melting point, Boiling point) do the molecules or atoms free themselves from interatomic forces of attraction and conversion of a state i.e. phase change takes place.
Heat energy of a body can also be defined as a form of energy that can be transferred from one body to the other or within the body itself with a temperature difference and can be generated by a body at the expense of other forms of energy. The SI unit of heat energy is Joule abbreviated as ‘J’. In CGS system, however, heat is measured in ‘Calorie’ (Cal.) where 1 Calorie = 4.186 J
Browse more Topics under Thermal Properties Of Matter
- Calorimetry
- Change of State
- Ideal Gas Equation and Absolute Temperature
- Newton’s Law of Cooling
- Specific Heat Capacity
- Temperature and Heat
- Thermal Expansion
What is Temperature?
The temperature of a body is the measure of the amount of heat content possessed by it. It is measured in degree Celcius (°C) or Kelvin(°K). The temperature of a substance is a physical quantity that measures the degree of hotness or coldness of a body.
You can download Thermal Properties of Matter Sheet by clicking on the download button below

Thermal Properties of Matter
The properties of the matter involving heat transfer and measurement are known as ‘Thermal Properties of Matter’
Heat Transfer
Heat energy can be transferred from one body to the other or from one location in a body to the other. Study of the techniques and methods adopted to transfer heat energy is known as ‘Heat Transfer’. To facilitate heat transfer between 2 bodies there needs to be a temperature difference between them.This means that these bodies must be a 2 different temperatures one higher than the other to allow heat to flow from one body to the other.
This means that no heat transfer occurs between 2 bodies which are at the same temperature. At the same time, it is very important to note that heat only flows from a body at higher temperature to a body at a lower temperature. Although this may look obvious, this law is very important from the point of view of thermodynamics.
Case Study: Cup of Tea
Let us say that you have prepared a cup of tea for yourself. The tea is very hot say at 80°C and so you leave it in a room with a temperature of 25 ° C for some time to cool down. This is the first law of heat transfer. Heat transfer will only take place between 2 bodies when they have a substantial temperature difference.
Now, after some time you come back to find that the tea in the cup has cooled down to say 50°C and you have a sip of the same. This is the second law of heat transfer. Heat will only flow from a body at higher temperature to a body at a lower temperature. It is not possible to have a scenario where the heat flows from the room at 25° c to the cup of tea at 80° C and heat it even further.
These techniques and methods discussed below are observed in nature and thus have been generalized for all things while under consideration for the purpose of the study. However, no observation against these has been ever recorded or observed thus establishing their credibility as truthful and applicable at all times.
Heat transfer takes place in 1 of the three ways namely: Conduction, Convection and Radiation We will discuss each of these methods in detail.
Conduction
Conduction is the method of transfer of heat within a body or from one body to the other due to the transfer of heat by molecules vibrating at their mean positions. The bodies through which the heat transfer must be in contact with each other. There is no actual movement of matter while transferring heat from one location to the other.
Conduction occurs usually in solids where molecules in the structure are held together strongly by intermolecular forces of attraction amongst them and so they only vibrate about their mean positions as they receive heat energy and thus pass it to the surrounding molecules by vibrations.
Convection
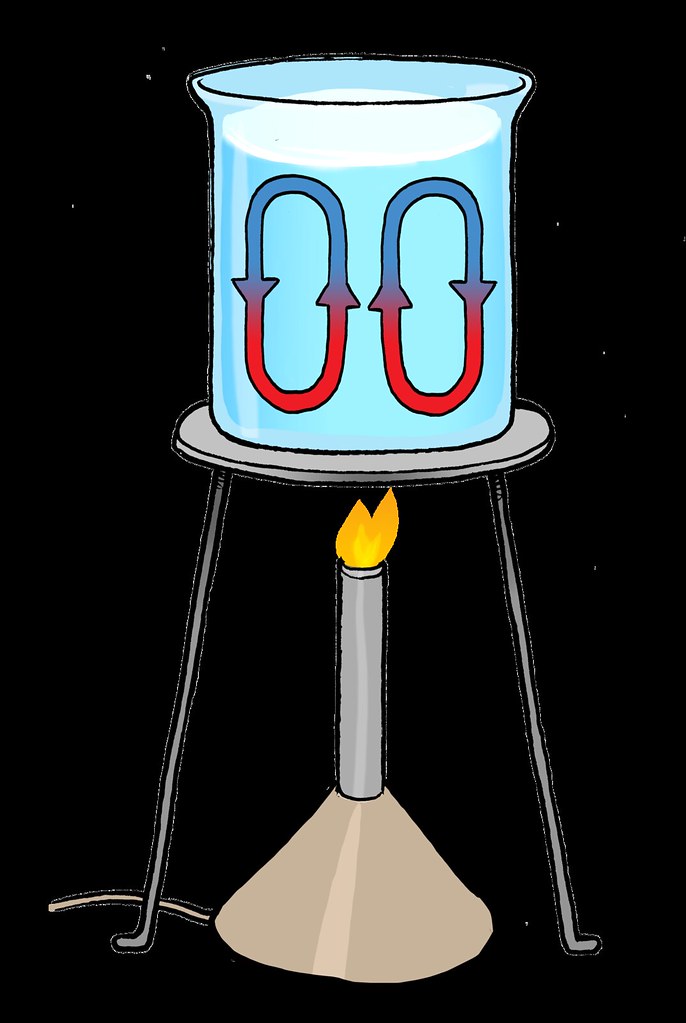
Convection is the mode of heat transfer which occurs mostly in liquids and gases.In this method, heat transfer takes place with the actual motion of matter from one place within the body to the other. Often when we boil water we have seen bubbles and currents develop in the water on careful observation.
This is an apt example of the convection process. The hot water at the bottom becomes lighter and moves upwards forcing the cold and denser water at the top to come down and thus get heated up.
Radiation
Radiation is another form of heat transfer. It does not require any medium and can be used for transfer of heat in a vacuum as well. This method uses electromagnetic waves which transfer heat from one place to the other. The heat and light from the sun in our solar system reach our planet using radiation only.
In fact, radiation is the most potent method of heat transfer. In winters when we sit near a fire we feel warm without actually touching the burning wood. This is possible by radiation only.
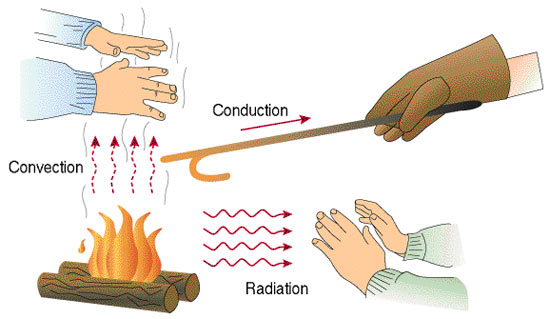
The above example effectively demonstrates the 3 methods of heat transfer we have discussed above. Another important point while discussing heat transfer is as follows:
Suppose an object has to be cooled down in relation to the ambient temperature. How would you determine the amount of time required for a given article to acquire the ambient temperature? The answer to that is given by ‘Newton’s law of Cooling’.
Newton’s Law of cooling
Newton’s law of Cooling states that there is always a direct correlation between the rate of change of temperature of a body and the temperature difference between the object and the surroundings. What this simply means is a hot body will cool down faster if the temperature of the body is much greater than that of the ambient. Whereas another body which has a temperature that is closer to the ambient temperature will take a little more time.
For example, A cup of tea at 80° Celcius loses heat at a much faster rate to a surrounding at a temperature of 25°Celcius as compared to a cup of tea which is at 35° Celcius. This brings us to the end of our discussion. A thorough knowledge of these concepts will go a long way to help the reader understand concepts of heat transfers, thermodynamics, and their applications as well.
Solved Examples for You
Question: Two rods of the same length and transfer a given amount of heat 12 Second, when they are joined as shown in figure (i) But When they tire joined as shown in figure (ii), then they will transfer same heat in same. conditions in

- 24 s
- 12 s
- 6 s
- 48 s
Solution: Option (D) 48 s. We know that heat flow is given as: Q = \( \frac{KA \Delta T}{L} \)
If they are connected parallel to each other, t α \( \frac{L}{A} \)
Now, if they are connected in series then: t’ α \( \frac{2L}{\frac {A}{2}} \)
So, t’ = 4 × t = 4 × 12 = 48 seconds






Leave a Reply