What are enzymes and what do they do in our bodies? Enzymes are basically proteins that are produced by living organisms to bring about certain metabolic and biochemical reactions in the body. They are biological catalysts that speed up reactions inside the body. Let’s find out more about them.
Suggested Videos:
What is the Structure of Enzymes?
Enzymes, as mentioned above, are biological catalysts. While they hasten or speed up a process, they are actually providing an alternative pathway for the process. But, in the process, the structure or composition of the enzymes remain unaltered.
Enzymes are actually made up of 1000s of amino acids that are linked in a specific way to form different enzymes. The enzyme chains fold over to form unique shapes and it is these shapes that provide the enzyme with its characteristic chemical potential. Most enzymes also contain a non-protein component known as the co-factor.
Browse more Topics under Biomolecules
- Biomacromolecules
- Bond linking Monomers
- Enzymes
- Metabolic Basis For Living
- Nucleic Acids
- Polysaccharides
- Proteins
Types of Enzymes:
The biochemical reactions occurring in the body are basically of 6 types and the enzymes that bring about these reactions are named accordingly:
- Oxidoreductases: These enzymes bring about oxidation and reduction reactions and hence are called oxidoreductases. In these reactions, electrons in the form of hydride ions or hydrogen atoms are transferred. When a substrate is being oxidized, these enzymes act as the hydrogen donor. These enzymes are called dehydrogenases or reductases. When the oxygen atom is the acceptor, these enzymes are called oxidases.
- Transferases: These enzymes are responsible for transferring functional groups from one molecule to another. Example: alanine aminotransferase which shuffles the alpha‐amino group between alanine and aspartate etc. Some transferases also transfer phosphate groups between ATP and other compounds, sugar residues to form disaccharides such as hexokinase in glycolysis.
- Hydrolases: These enzymes catalyze reactions that involve the process of hydrolysis.They break single bonds by adding water. Some hydrolases function as digestive enzymes because they break the peptide bonds in proteins. Hydrolases can also be a type of transferases as they transfer the water molecule from one compound to another. Example: Glucose-6-phosphatase that removes the phosphate group from glucose-6-phosphate, leaving glucose and H3PO4.
- Lyases: These enzymes catalyze reactions where functional groups are added to break double bonds in molecules or where double bonds are formed by the removal of functional groups. Example: Pyruvate decarboxylase is a lyase that removes CO2 from pyruvate. Other examples include deaminases and dehydratases.
- Isomerases: These enzymes catalyze the reactions where a functional group is moved to another position within the same molecule such that the resulting molecule is actually an isomer of the earlier molecule. Example: triosephosphate isomerase and phosphoglucose isomerase for converting glucose 6-phosphate to fructose 6-phosphate.
- Ligases: These enzymes perform a function that is opposite to that of the hydrolases. Where hydrolases break bonds by adding water, ligases form bonds by removal of the water component. There are different subclasses of ligases which involve the synthesis of ATP.
How do enzymes work?
For any reaction to occur in the universe, there is an energy requirement. In cases where there is no activation energy provided, a catalyst plays an important role to reduce the activation energy and carried forward the reaction. This works in animals and plants as well. Enzymes help reduce the activation energy of the complex molecules in the reaction. The following steps simplify how an enzyme works to speed up a reaction:
Step 1: Each enzyme has an ‘active site’ which is where one of the substrate molecules can bind to. Thus, an enzyme- substrate complex is formed.
Step 2: This enzyme-substrate molecule now reacts with the second substrate to form the product and the enzyme is liberated as the second product.
There are many theories that explain how enzymes work. But, there are two important theories that we will discuss here.
Theory 1: Lock and Key Hypothesis
This is the most accepted of the theories of enzyme action.

This theory states that the substrate fits exactly into the active site of the enzyme to form an enzyme-substrate complex. This model also describes why enzymes are so specific in their action because they are specific to the substrate molecules.
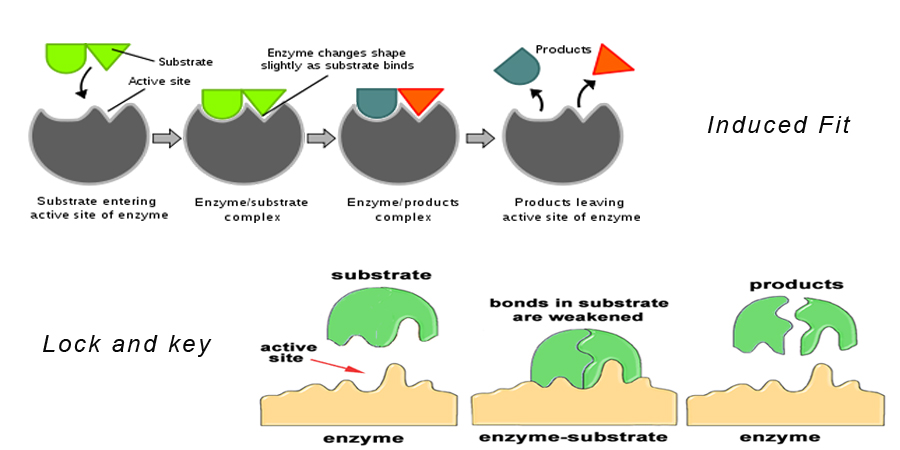
Theory 2: Induced Fit Hypothesis
This is similar to the lock and key hypothesis. It says that the shape of the enzyme molecule changes as it gets closer to the substrate molecule in such a way that the substrate molecule fits exactly into the active site of the enzyme.
What factors affect enzyme activity in the cell?
- Concentration of Enzymes and Substrates: The rate of reaction increases with increasing substrate concentration up to a point, beyond which any further increase in substrate concentration produces no significant change in reaction rate. This occurs because after a certain concentration of the substrate, all the active sites on the enzyme are full and no further reaction can occur.
- Temperature: With the increase in temperature, the enzyme activity increases because of the increase in kinetic energy of the molecules. There is an optimum level when the enzymes work at the best and maximum. This temperature is often the normal body temperature of the body. When the temperature increases beyond a certain limit, enzymes, which are actually made up of proteins, begin to disintegrate and the rate of reaction slows down.
- pH: Enzymes are very sensitive to changes in the pH and work in a very small window of permissible pH levels. Below or above the optimum pH level, there is a risk of the enzymes disintegrating and thereby the reaction slows down.
- Inhibitors: Presence of certain substances that inhibit the action of a particular enzyme. This occurs when the inhibiting substance attaches itself to the active site of the enzyme thereby preventing the substrate attachment and slows down the process.
Solved Example for You
Q: An enzyme acts by?
a. Increasing the energy of activation
b. Decreasing the energy of activation
c. Decreasing the pH
d. Increasing the pH
Sol: a. Increasing the energy of activation
The reactants do not undergo chemical change automatically. They do so in the transition state. Transition state has more free energy than reactants or products. The inability of reactants to undergo change due to the requirement of extra energy for converting them to transition state is called as ‘Energy Barrier’. The energy required to overcome energy barrier is called as ‘Activation Energy’.






DNA consists of thymine or thiamine??