Did you ever enter a washroom that smelled so bad? What is that smell of? That smell is of nothing but ammonia. It is a compound of nitrogen, which has a pungent smell but it is of great importance to humans. From fertilizers to dyes, it is useful in day-to-day life. Let’s learn more about this important compound.
Suggested Videos
What is Ammonia?
Ammonia (NH3) is an essential compound of nitrogen and hydrogen. It is created by the regular decay of vegetable and animal bodies. The demise and rot of animals and plants cause the nitrogen compounds exhibit in them to get deteriorated, producing ammonia. Ammonia, likewise, is present in the soil as ammonium salts.
Preparation
We can manufacture the compound by the following methods:
1) From Ammonium Chloride
We can generate ammonia gas in the research centre by slowly heating ammonium chloride (NH4Cl) and slaked lime [Ca(OH)2].
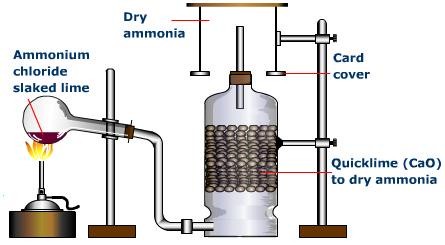
Ammonia gas is lighter than air, requiring its accumulation by the descending displacement of air. Since it is quite solvent in water it can’t be gathered over it.
Advancing ammonia gas through quicklime (CaO) dries it. Being an essential gas, we can’t dry it by advancing it through concentrated sulphuric acid or phosphorus pentoxide (P2O5). This is because it reacts with them to frame ammonium sulfate or ammonium phosphate separately.
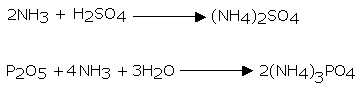
We can’t utilise calcium chloride for drying ammonia gas as it forms ammoniates with CaCl2.
![]()
2) By the Hydrolysis of Metal Nitrides
Hydrolyzing metal nitrides like magnesium and aluminium nitrides, with water or alkalis, can likewise deliver ammonia gas.
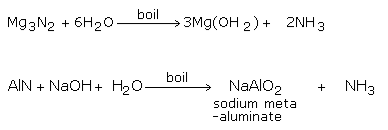
Structure
Ammonia is a covalent atom. It is seen as a dot structure. The particle is shaped because of the overlap of orbitals of three hydrogen atoms and three sp3 hybrid orbitals of nitrogen in the structure as the central atom. The fourth sp³ hybrid orbital is involved by a lone pair.
This provides a trigonal pyramidal shape to the compound. The H-N-H bond edge is 107.3°, which is somewhat not exactly the tetrahedral edge of 109°28. This is on the grounds that the bond pair-lone pair repulsions push the N-H bonds somewhat inwards. In solid and liquid states, ammonia is related through hydrogen bonds.

Physical Properties
Ammonia is a gas. It has no colour. It has a sharp pungent odour having a soapy taste. At the point when inhaled all of a sudden, it attacks the eyes bringing tears. It is lighter than air. It is very soluble in water. Ammonia effortlessly melts at room temperature at a pressure of around 8-10 atmospheres.
Liquid ammonia bubbles at 239.6 K (- 33.5°C) under one-atmosphere pressure. It has a high value of the latent heat of vaporization (1370 J for each gram). It solidifies at 195.3 K (- 77.8°C) to give a solid that is white crystalline in appearance.
Chemical Properties
1) Thermal Stability
Ammonia is exceptionally inert. In any case, we can disintegrate it into hydrogen and nitrogen by advancing over metallic impetuses that have been heated.
![]()
2) Combustibility
It is flammable in air.
![]()
3) Basic Character
The compound has a natural propensity to give its lone pair of electrons of nitrogen to different atoms. Consequently, it acts like a strong Lewis base.
Uses
- We use it in the production of urea and rayon.
- We also use it in the production of composts, for example, ammonium nitrate, urea diammonium phosphate, ammonium sulfate and so on.
- More frequently, we also use it as a refrigerant, in ice plants.
- It finds its use in the furniture industry, as a purging operator for furniture and glass surfaces.
- We use it in the production of nitric acid by Ostwald’s procedure.
- We also use it in the production of sodium carbonate by Solvay’s procedure.
Tests
The undermentioned tests of any specimen affirm the presence of the compound:
- The ammoniacal odour of the compound is effectively perceivable having a trademark pungent smell.
- It turns wet red litmus blue and moist turmeric paper brown in colour.
- A glass bar dunked in concentrated HCl when conveyed near ammonia causes thick white exhaust.
- When added to a solution of copper sulphate, ammonia turns the solution deep blue.
NH3 + CuSO4 + nH2O→ [Cu(NH3)4(H2O)n]SO4
- When added to Nessler’s reagent (basic arrangement of K2[HgI4], ammonia gives a precipitate brown in colour.
NH4+ + 2[HgI4]2− + 4OH− → HgO·Hg(NH2)I ↓ + 7I− + 3H2O
Solved Example for You
Q: How can we make the ammonia converter?
Ans: We produce the converter using chrome-vanadium steel. It is normally 1.3 meters high and 1 meter in measurement. The converter is furnished with a warmth exchanger in the upper part and the impetus is packed in the focal segment of the converter.
There is a process to warm the combination of gases. After the gas blend enters through the bay at the base, the gases course around the impetus kept up at 450-500°C and afterwards goes through to the heat exchanger. The gases at the end enter the chamber of catalyst to give ammonia.
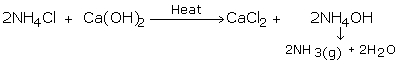






Leave a Reply