Ozone is important for us. Isn’t it? You know how it protects us from the harmful UV rays of the sun. We all use sunscreen with UV protection, don’t you? So, ozone gives you a natural sunscreen! In this chapter, we will look at ozone in greater detail. We will have a look at its properties and other uses as well.
Suggested Videos
What Exactly is Ozone?
Ozone is an allotrope of oxygen. It is highly unstable in nature. We can find its traces for about 20 kilometres above the sea level. So, how is ozone present at such heights? It is formed by the reaction of oxygen with the ultraviolet rays of the sun. The major role of this gas is protecting the earth’s surface from the harmful ultraviolet radiations from the sun.
You have heard of the depletion of the ozone layer. You know why is that? We use chlorofluorocarbons in refrigerators and other aerosols also that release harmful things into the air leading to gaps in the layer. Due to this, we get the UV light and it causes a lot of skin problems and malignancy in people.
Apart from this, the various oxides of nitrogen, particularly nitric oxide, react very rapidly with ozone to produce oxygen and nitrogen dioxide. Hence, the various nitrogen oxides of nitrogen that appear from the fumes frameworks of supersonic fly planes lead to depleting the layer.

Preparation
We can prepare ozone by passing a silent electric discharge through dry, unadulterated, and cold oxygen in an extraordinary device. This device is what we know as the ozoniser. In this process, we obtain the gas of up to 10% concentration. This is an endothermic process and we must take care to complete it at high temperatures. That is why we use a silent electric discharge.
If we want to produce higher concentrations of ozone, we can do so by the process of fractional liquefaction of an oxygen and ozone mixture.
Browse more Topics under The P Block Elements
- Introduction to p Block Elements
- Some Important Compounds of Carbon and Silicon
- Trend and Anomalous Properties of Carbon
- Trends and Properties of Boron and Aluminium
- Ammonia
- Chlorine
- Dinitrogen
- Dioxygen
- Group 13 Elements: Boron Family
- Group 14 Elements: Carbon Family
- Group 15 Elements
- Group 16 Elements
- Group 17 Elements
- Group 18 Elements
- Hydrogen Chloride
- Interhalogen Compounds
- Nitric Acid and Oxides of Nitrogen
- Oxoacids of Halogens
- Oxoacids of Phosphorus
- Oxoacids of Sulphur
- Phosphine
- Phosphorus – Allotropic Forms
- Phosphorus Halides
- Simple Oxides
- Sulphur – Allotropic Forms
- Sulphuric Acid
- Sulphuric Dioxide
Physical Properties
- Ozone is a gas with a light blueish colour.
- It has a fishy smell.
- It condenses at – 120°C to give a dull blue fluid. On further cooling, it hardens to give dark violet crystals.
- Thermodynamically, it is very unstable and disintegrates to oxygen. This is an exothermic process and is catalyzed by numerous materials. However, we must know that high concentration of the gas can be very dangerous.
Oxidizing Action
- Ozone is taken as a very strong oxidizing agent. This is mainly because of the ease with which it gives out atoms of nascent oxygen. It helps in oxidising:
- Lead sulphide to lead sulphate:
4O3 + PbS → 4O2 + PbSO4
- Iodide ions to iodine:
2KI + H2O + O3 → 2KOH + I2 + O2
- We use this particular reaction in the process of quantitative estimation of the gas. When we bring ozone in contact with potassium iodide solution with a borate buffer (pH 9.2), it liberates iodine. We can titrate this liberated iodine against a standard solution of sodium thiosulphate using starch as an indicator. The reactions that involve in this process are:
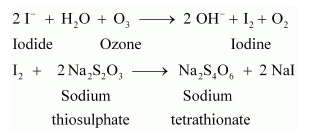
- It also helps in oxidising nitrogen dioxide to dinitrogen pentoxide.
2NO2 + O3 → N2O5 + O2
Solved Example For You
Q: Mention some uses of ozone.
Ans: The common uses of ozone include:
- We use it as an antiseptic and also as a disinfectant.
- We also use it as a delicate dying agent for fading oils, starch etc.






Leave a Reply