Phosphine (PH3) is a chemical compound which is prepared by heating phosphorous acid or also by reacting calcium phosphide with water. Phosphine finds its place in the group of organophosphorus compounds with the chemical formula of PH3. Philippe Gengembre discovered it in 1783. It can be found in human tissues, blood, urine, saliva, etc. Let us look at this chemical in greater detail in this chapter. We will see its physical properties, chemical properties and uses as well. Let’s begin.
Suggested Videos
What is Phosphine?
Phosphine is a chemical that finds its place in the group of organophosphorus compounds. Philippe Gengembre discovered or acquired this chemical in the year 1783. He was the one who acquired phosphine by heating phosphorous in an aqueous solution of potassium carbonate.
Phosphine carries the chemical formula of PH3. The concentration of this compound constantly alters in our environment. As we already mentioned, this chemical plays an important role in the phosphorous biochemical cycle.
Browse more Topics under The P Block Elements
- Introduction to p Block Elements
- Some Important Compounds of Carbon and Silicon
- Trend and Anomalous Properties of Carbon
- Trends and Properties of Boron and Aluminium
- Ammonia
- Chlorine
- Dinitrogen
- Dioxygen
- Group 13 Elements: Boron Family
- Group 14 Elements: Carbon Family
- Group 15 Elements
- Group 16 Elements
- Group 17 Elements
- Group 18 Elements
- Hydrogen Chloride
- Interhalogen Compounds
- Nitric Acid and Oxides of Nitrogen
- Oxoacids of Halogens
- Oxoacids of Phosphorus
- Oxoacids of Sulphur
- Ozone
- Phosphorus – Allotropic Forms
- Phosphorus Halides
- Simple Oxides
- Sulphur – Allotropic Forms
- Sulphuric Acid
- Sulphuric Dioxide
Preparation of Phosphine
(i) From Phosphide
We can obtain the compound by reacting phosphides with water. For example, calcium phosphide reacts with water to give calcium hydroxide and phosphine.
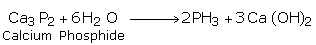
(ii) From Phosphorus Acid
We can also obtain the compound by heating phosphorus acid. It decomposes to give a pure sample of phosphine.
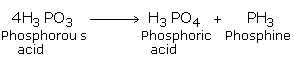
Structure of Phosphine
You can see that the electronic configuration of phosphine resembles ammonia. It has the structure of a pyramid. The bond angle H-P-H is = 93°. On the other hand, ammonia has pyramidal geometry with a bond angle of 107.80. Thus, we see that both these chemicals have a comparative bond angle. Phosphorus is less electronegative than nitrogen.
The cloud of electrons around the central atom, phosphorus is less concentrated as compared to the nitrogen present in ammonia. Therefore, the lone pair of electrons causes significantly more contortion in PH3. Thus, we note the decline in the bond angle in PH3 to 93.5°.

Physical and Chemical Properties of Phosphine
- Phosphines are colourless gas.
- It has a characteristic smell like that of a spoiled fish.
- It is an exceedingly noxious gas. PH3 is sparingly dissolvable in water. However, it can dissolve in natural solvents.
- PH3 acts as a Lewis base by giving away its lone pair of electrons by reacting with hydrogen iodide.
- Under typical conditions, it is a non-ignitable gas. But, when you warm it, it bursts into flames, forming phosphoric acid.
- It explodes violently when we expose it to oxidising agents.
Effects of PH3
Phosphine is an extremely dangerous gas. Exposure to even little quantities of the gas can lead to dizziness, loose bowels, cough, cerebral pain, and chest tightness, just to name a few. Upon a greater exposure, you have the risk of suffering from convulsions, coma, damage to the kidney and liver and irregular heartbeat.
Properties of PH3 Ligands
- Tertiary phosphines or PR3, are an important class of ligands. This is mainly due to the fact that we can change their electronic and steric properties in a very orderly path over a wide range by just shifting the R group(s).
- These are capable of stabilising a completely wider array of metal complexes that might interest organometallic scientific expert as their phosphine complexes (R3P) nM−L.
- Phosphines are usually spectator ligands and not performer ligands.
- Like NR3, phosphines also possess a lone pair on the focal particle which it gives to a metal.
- For alkyl phosphines, the π acidity is very weak.
Solved Example for You
Q: Write down the various uses of phosphine.
Ans: The various uses of phosphine include:
- We use phosphine as a dopant, in the semiconductor industries.
- PH3 plays an important part in the Holme’s signal. This is because it can rapidly ignite. The compartments having calcium carbide and calcium phosphide are penetrated and dropped into the ocean when the gasses develop smoulder. They help in serving as a signal to the Mariners.
- It is also an essential part of smoke screens. Containers having a punctured base and a gap at the top are recorded with calcium phosphide and calcium carbide. These are, then, let out into the ocean. Water enters the containers through the base. It reacts and produces acetylene and phosphine.
- Phosphine gets ignited immediately as it interacts with air. As a further step, it also incites the ignition in acetylene. Due to these reactions, we get a bright and nice red fire. This has a huge cloud of dense smoke due to the smouldering of phosphine. This helps in giving a signal to the approaching boats.
- Phosphine fumigants are a common ingredient in the households to control the bugs, rodents and rabbit invasion in a huge range of stored grains. Human beings can recognise the scent of PH3 easily as it is quite strong. However, the rodents and other bugs or insects can’t know its presence easily. Thus, it helps in driving or killing them away. We transport PH3 as compressed liquefied gas. A few solids (phosphides) discharge PH3 gas.






Leave a Reply