Have you ever seen someone look similar to you? Too often, we come across people who look “exactly like” someone you know. However, they are NOT the same person! Such is the case with allotropes. Did you know about this? In this chapter, we will cover the various allotropes of phosphorus and look at their forms and properties. However, let us start with the basics of what allotropes are.
Suggested Videos
What is an Allotrope?
Allotropy is the science of occurrence of an element in multiple or more than one physical shapes. Allotropes are the various physical types of the same element. A lot of elements exhibit the property of allotropy. These allotropes show different physical properties. However, their chemical properties are comparable.
Browse more Topics under The P Block Elements
- Introduction to p Block Elements
- Some Important Compounds of Carbon and Silicon
- Trend and Anomalous Properties of Carbon
- Trends and Properties of Boron and Aluminium
- Ammonia
- Chlorine
- Dinitrogen
- Dioxygen
- Group 13 Elements: Boron Family
- Group 14 Elements: Carbon Family
- Group 15 Elements
- Group 16 Elements
- Group 17 Elements
- Group 18 Elements
- Hydrogen Chloride
- Interhalogen Compounds
- Nitric Acid and Oxides of Nitrogen
- Oxoacids of Halogens
- Oxoacids of Phosphorus
- Oxoacids of Sulphur
- Ozone
- Phosphine
- Phosphorus Halides
- Simple Oxides
- Sulphur – Allotropic Forms
- Sulphuric Acid
- Sulphuric Dioxide
Allotropes of Phosphorus
In this chapter, we will look at the allotropes of phosphorus. This element exists in a few allotropic forms. The main allotropes of phosphorus include the white phosphorus, red phosphorus and black phosphorus. In addition to these, there also exists a violet phosphorus. However, that is not a significant allotrope. So, let us start with the various allotropes of phosphorus now.
White Phosphorus
It is a common allotrope of phosphorus. White phosphorus is a waxy and translucent solid. It is very delicate and needs proper handling. It is insoluble in water. However, it dissolves in carbon disulphide or carbon tetrachloride. It breaks down in boiling caustic soda in a latent air and produces sodium hypophosphite and phosphine.
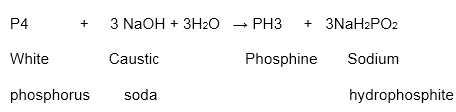
Structure of White Phosphorus

In the above diagram, we see the structure of white phosphorus. As we can see, it has a tetrahedral shape. Every phosphorus particle has a covalent bond with three different atoms of phosphorus. There exist weak Van Der Waals forces of attraction between these particles. We must remember that this element is very reactive and too harmful.
- The melting point is quite low at 44°C.
- As we see, the bond angle in a P4 particle is 60°. This is comparatively very less as compared to a normal bond angle or a hypothetical bond angle. Therefore, it has a strain in itself. This is why white phosphorus is highly unstable and reactive.
- White phosphorus catches fire suddenly in the air at around 35°C. As you can notice, this temperature is marginally higher than the normal room temperature. This is the reason why it is kept in water. After combustion, it produces phosphorus pentoxide.
P4 + 5O2 → 2P2O5 or P4O10
- When it comes in contact with moist air, white phosphorus undergoes an oxidation reaction. This reaction leads to a sparkling discharge of light. As an outcome, it sparkles oblivious.
- White phosphorus displays chemiluminescence.
Red Phosphorus
- We can obtain red phosphorus by heating white phosphorus to around 250°Celsius within the sight of daylight.
- Red phosphorus is iron-grey in colour. It is a radiant and bright crystalline solid.
- It is non-poisonous and does not have any odour. Red Phosphorus does not dissolve I water and also in carbon tetrachloride.
- It doesn’t break up in boiling caustic soda-like white phosphorus. In fact, it disintegrates in alcoholic potash.
- We can find it in the state of a polymeric solid.

- It is steady under ordinary conditions and doesn’t catch fire in the air.
- However, it experiences burning when we warm it to around 400°C.
- Red phosphorus doesn’t show chemiluminescence.
Black Phosphorus
- We can prepare black phosphorus from white phosphorus by heating it to 470K at inert temperature.
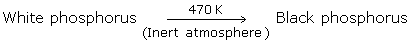
- Black phosphorus is the most stable allotrope of phosphorus. It has a layered structure. It is a very highly polymerised form of the element.
- We can find black phosphorus in two main forms. They are alpha black phosphorus and beta black phosphorus.
- While beta black phosphorus forms when white phosphorus is heated at 473K, alpha black phosphorus forms when we heat red phosphorus at 803K.
- Beta black phosphorus conducts electricity while alpha black phosphorus doesn’t conduct electricity.
Uses of Phosphorus
Phosphorus compounds assume a vital part of life forms. Phosphorus forms a basic constituent in the animal and plant matter.We find it present in blood, bones and the brain of all the animals and also, in living cells. A few of its compounds find applications in industries. The most essential of these chemicals are orthophosphoric acid and phosphatic composts.
Solved Examples For You
Q1: What is yellow phosphorus?
Ans: We use the term yellow phosphorus to denote white phosphorus. It is an allotrope of phosphorus.
Q2: Describe the structure of alpha black phosphorus and beta black phosphorus.
Ans: We find alpha black phosphorus in the shape of opaque monoclinic crystals. We might also see rhombohedral crystals for the same. On the other hand, beta black phosphorus is available as corrugated sheets. This is why they have a structure consisting of flaky and layered crystals. The difference in their structure is also a reason why beta black phosphorus can conduct electricity while the other can not.






Leave a Reply