In 1911, Rutherford, along with his assistants, H. Geiger and E. Marsden, performed the Alpha Particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’ – a major step towards how we see the atom today.
Suggested Videos
J.J Thomson’s Plum-pudding Model
In 1897-98, the first model of an atom was proposed by J.J. Thomson. Famously known as the Plum-pudding model or the watermelon model, he proposed that an atom is made up of a positively charged ball with electrons embedded in it. Further, the negative and positive charges were equal in number, making the atom electrically neutral.
Figure 1 shows what Thomson’s plum-pudding model of an atom looked like. Ernest Rutherford, a former research student working with J.J. Thomson, proposed an experiment of scattering of alpha particles by atoms to understand the structure of an atom.
Rutherford, along with his assistants – H. Geiger and E. Marsden – started performing experiments to study the structure of an atom. In 1911, they performed the Alpha particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’ – a major step towards how we see the atom today.

Figure 1. Source: Wikipedia
Browse more Topics under Atoms
The Alpha Particle Scattering Experiment
They took a thin gold foil having a thickness of 2.1×10-7 m and placed it in the centre of a rotatable detector made of zinc sulfide and a microscope. Then, they directed a beam of 5.5MeV alpha particles emitted from a radioactive source at the foil. Lead bricks collimated these alpha particles as they passed through them.
After hitting the foil, the scattering of these alpha particles could be studied by the brief flashes on the screen. Rutherford and his team expected to learn more about the structure of the atom from the results of this experiment.
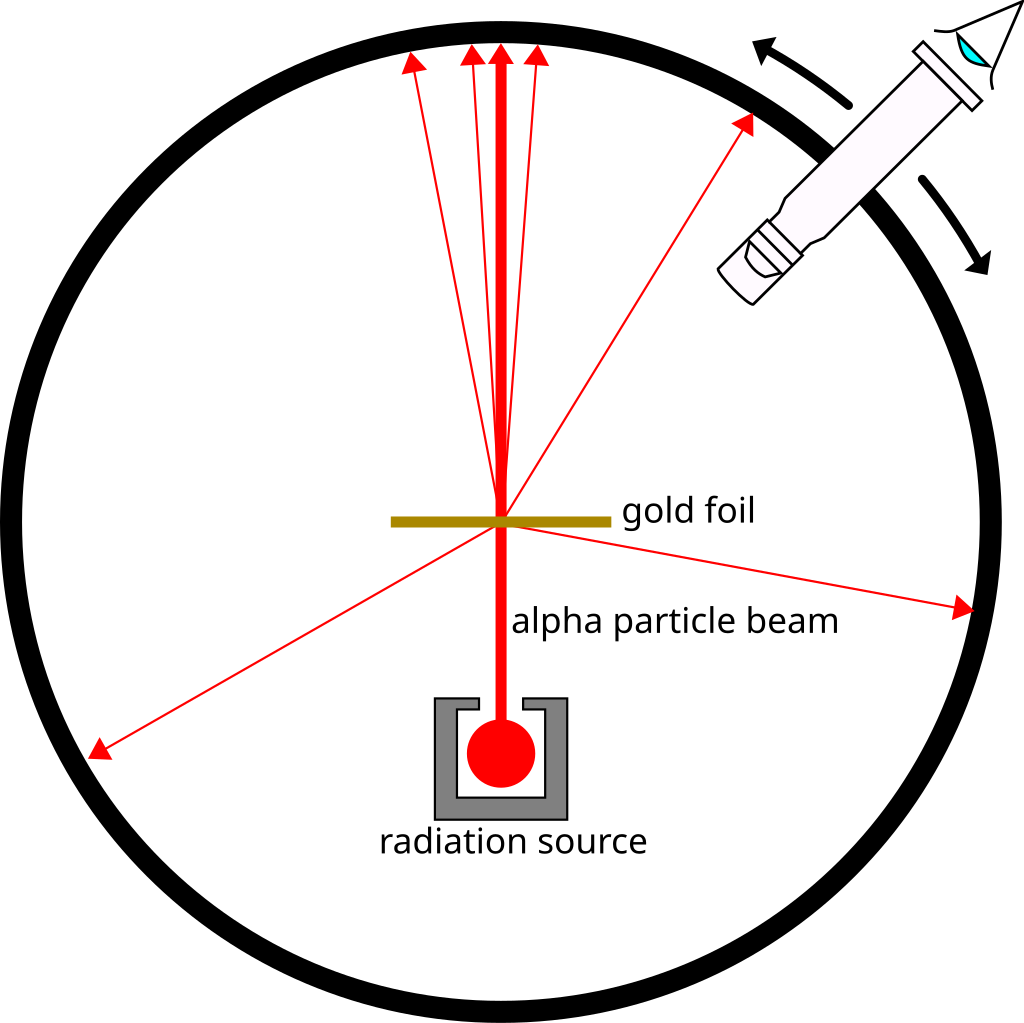
Source: Wikipedia
Observations
Here is what they found:
- Most of the alpha particles passed through the foil without suffering any collisions
- Around 0.14% of the incident alpha particles scattered by more than 1o
- Around 1 in 8000 alpha particles deflected by more than 90o
These observations led to many arguments and conclusions which laid down the structure of the nuclear model on an atom.
Conclusions and arguments
The results of this experiment were not in sync with the plum-pudding model of the atom as suggested by Thomson. Rutherford concluded that since alpha particles are positively charged, for them to be deflected back, they needed a large repelling force. He further argued that for this to happen, the positive charge of the atom needs to be concentrated in the centre, unlike scattered in the earlier accepted model.
Hence, when the incident alpha particle came very close to the positive mass in the centre of the atom, it would repel leading to a deflection. On the other hand, if it passes through at a fair distance from this mass, then there would be no deflection and it would simply pass through.
He then suggested the ‘nuclear model of an atom’ wherein the entire positive charge and most of the mass of the atom is concentrated in the nucleus. Also, the electrons are moving in orbits around the nucleus akin to the planets and the sun. Further, Rutherford also concluded from his experiments that the size of the nucleus is between 10-15 and 10-14 m.
According to Kinetic theory, the size of an atom is around 10-10 m or around 10,000 to 100,000 times the size of the nucleus proposed by Rutherford. Hence, the distance of the electrons from the nucleus should be around 10,000 to 100,000 times the size of the nucleus.
This eventually implies that most of the atom is empty space and explains why most alpha particles went right through the foil. And, these particles are deflected or scattered through a large angle on coming close to the nucleus. Also, the electrons having negligible mass, do not affect the trajectory of these incident alpha particles.
Alpha Particle Trajectory
The trajectory traced by an alpha particle depends on the impact parameter of the collision. The impact parameter is simply the perpendicular distance of each alpha particle from the centre of the nucleus. Since in a beam all alpha particles have the same kinetic energy, the scattering of these particles depends solely on the impact parameter.
Hence, the particles with a small impact parameter or the particles closer to the nucleus, experience large angle of scattering. On the other hand, those with a large impact parameter suffer no deflection or scattering at all. Finally, those particles having ~zero impact parameter or a head-on collision with the nucleus rebound back.
Coming to the experiment, Rutherford and his team observed that a really small fraction of the incident alpha particles was rebounding back. Hence, only a small number of particles were colliding head-on with the nucleus. This, subsequently, led them to believe that the mass of the atom is concentrated in a very small volume.
Electron Orbits
In a nutshell, Rutherford’s nuclear model of the atom describes it as:
- An electrically neutral sphere with
- A small and positively charged nucleus at the centre
- Surrounded by revolving electrons in their dynamically stable orbits
The centripetal force that keeps the electrons in their orbits is an outcome of:
- The electrostatic force of attraction between-
- The positively charged nucleus and
- The negatively charged revolving electrons.
Solved Example for You
Question: Rutherford, Geiger and Marsden, directed a beam of alpha particles on a foil of which metal
- Platinum
- Tungsten
- Gold
- Silver
Solution: Gold






i really have learnt alot, but its is difficult for me to register because my country(Nigeria) is not on the listed countries. pls kindly include if you can