Electrons are negatively charged and have their own energy. The energy of an electron defines which orbit it will be in. In this topic, we will learn about the Energies of Orbitals, factors affecting them and lot more.
Browse more Topics Under Structure Of Atom
- Introduction: Structure of Atom
- Atomic Number
- Bohr’s Model of Atom
- Charged Particles in Matter
- Isobars
- Isotopes
- Mass Number
- Neutrons
- Rutherford’s Model of an Atom
- Thomson’s Model of an Atom
- Valency
- How are Electrons Distributed in Different Orbits (Shells)?
- Sub-Atomic Particles
- Atomic Models
- Shapes of Atomic Orbitals
- Energies of Orbitals
- Quantum Numbers
- Development Leading to Bohr’s Model of Atom
- Emission and Absorption Spectra
- Towards Quantum Mechanical Model of Atom
Suggested Videos
Energies of Orbitals
The energy which is essential to take an electron present in that orbital to infinity or the release of energy when an electron from an infinity it is added to that orbital, it is referred to as the energy of orbitals.
This orbital energy is dependent upon the principle of the quantum number (n) as well as the azimuthal quantum number (l) which is that it depends on the shell and subshells. For all those orbitals that belong to the same subshell, it is the same and those orbitals that are with the same energy are stated as degenerate orbitals.
The order of the increase in energy along the various orbitals is stated as –
1s < 2s = 2p < 3s = 3p = 3d < 4s = 4p = 4d = 4f
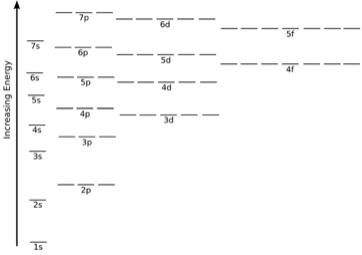
Factors affecting the Orbital Energy
- The s orbital electron will be more tightly bound to the nucleus as compared to the p orbital electron, which is more tightly bound in regard to a d orbital electron for a given value of the principal quantum number.
- As compared to p orbital electrons, s orbital electrons will have more negative or lesser amount of energy. Here, the p orbital electrons will have lesser energy than that of d orbital electrons.
- As the extent of shielding from the nucleus is different for the electrons in different orbitals, it leads to the splitting of energy levels that have the same principal quantum number. Therefore, the orbital energy would depend on the values of both the principal quantum number and azimuthal quantum number, symbolized as n and l respectively. Hence, the lower value of (n + 1) for an orbital, the lower is its energy.
- With the increase in the atomic number (Zeff), the orbital energy decreases in the same subshell.
The energies of orbitals of hydrogen and hydrogen-like particles are dependent upon the value of the principal quantum (n) numbers only as well as those of multi-electron atoms that depend upon the principal quantum number (n) as well as the azimuthal quantum number (l). Energy level diagrams are the diagram that represents the orbitals arrangement in order of their increasing energies.
You can download Structure of Atom Cheat Sheet by clicking on the download button below

Some Important Observations
The important observations derived from the energy level diagrams of multi-electron atoms are
- The subshell of a particular shell does not have equal energies. For example – 2s and 2p have different energies.
- In a particular shell, the subshell that holders the lower value of I has the lower energy. In the 2nd shell, 2s (I = 0) has a lower energy than 2p (I = 1). However, in the shell 3, energy is placed in order as – 3s < 3p < 3d
- For the same value of n, the differences between the energies of s and p subshell are small whereas, between p and d subshell, it is large and so on.
- With the increase in the value of n, the subshell of the lower shell may have higher energy than that of a higher shell which means 3d has higher energy than 4s.
Solved Question for You
Question: What is the lowest value of n that allows g orbitals to exist?
Solution: For g-orbitals, l = 4.
As for any value ‘n‘ of principal quantum number, the Azimuthal quantum number (l) can have a value from zero to (n – 1).
For l = 4, minimum value of n = 5.







Leave a Reply