Oxides of Nitrogen are a mixture of seven different gases and compounds which are form from nitrogen and oxygen. Gases in this group are Nitrous Oxide (N2O), Nitrogen Monoxide (NO), Dinitrogen Trioxide (N2O3), Nitrogen Dioxide (NO2), Dinitrogen Pentoxide (N2O5), Dinitrogen Tetroxide (N2O4). Nitric Oxide and Nitrogen Dioxide are common and hazardous. Without nitrogen, life would hardly sustain. In this chapter, we will look at oxides of nitrogen and Nitric acid.
Suggested Videos
Nitric Acid
Nitric acid is the most popular and helpful oxoacid of nitrogen. It has a molecular formula of HNO3 and its molar mass is 63.01 g mol-1. Now, let us see the various properties of the nitric acid.
Browse more Topics under The P Block Elements
- Introduction to p Block Elements
- Some Important Compounds of Carbon and Silicon
- Trend and Anomalous Properties of Carbon
- Trends and Properties of Boron and Aluminium
- Ammonia
- Chlorine
- Dinitrogen
- Dioxygen
- Group 13 Elements: Boron Family
- Group 14 Elements: Carbon Family
- Group 15 Elements
- Group 16 Elements
- Group 17 Elements
- Group 18 Elements
- Hydrogen Chloride
- Interhalogen Compounds
- Oxoacids of Halogens
- Oxoacids of Phosphorus
- Oxoacids of Sulphur
- Ozone
- Phosphine
- Phosphorus – Allotropic Forms
- Phosphorus Halides
- Simple Oxides
- Sulphur – Allotropic Forms
- Sulphuric Acid
- Sulphuric Dioxide
Physical Properties of Nitric Acid
- Pure nitric acid is nothing but a colourless fuming fluid. It has a typical and strong odour.
- Upon standing, it creates a yellow shading. This is because of the presence of various oxides of nitrogen that are dissolved in it. The primary oxide is NO2.
- The acid is completely soluble in water.
- The thickness of the pure acid is 1.54 g/mL.
- We can see that anhydrous nitric acid bubbles at 355.6 K (83.6°C). It is capable of developing a white solid at 231.4 K (- 41.7°C).
- It can corrode the skin and causes yellow rashes.
Chemical Properties of Nitric Acid
In this section, we will cover some of the most important chemical properties of Nitric Acid.
1) Stability
In its pure form, Nitric acid is not totally stable. Even at normal temperatures, it slightly decays when we expose it to daylight. Upon strong heating, it breaks down and gives nitrogen dioxide, oxygen and water.
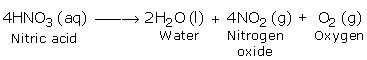
Nitrogen dioxide has a reddish brown colour. However, it might further break down in the undecomposed acid and produce its yellowish chestnut shade.
2) Acidic Nature
Nitric acid ionises easily in water and is a strong monobasic acid.
![]()
- Reactions with Basic Oxides: Nitric acid reacts with basic oxides to give a nitrate salt and water. For example, it reacts with calcium oxide to give calcium nitrate and water.
- Reaction with Bases (Hydroxides): Nitric acid reacts with hydroxides to give nitrate salt and water. For example, it reacts with sodium hydroxide to give sodium nitrate and water.
- Reaction with Carbonates and Hydrogen Carbonates: Nitric acid reacts with carbonates and hydrogen carbonates to give nitrate salt, carbon dioxide and water. For example, it reacts with sodium carbonate to give sodium nitrate, water and carbon dioxide.
- Reaction with Metals: Nitric acid does not behave like an acid with metals to form a salt and free hydrogen. However, magnesium and manganese are the two metals that react with cold and extremely dilute (1%) nitric acid to give out hydrogen.
Let us now look at the concept of various oxides of nitrogen.

Oxides of Nitrogen
Nitrogen reacts with oxygen and results in a number of nitrogen oxides. The oxidation states of all these oxides are pretty different. They are in the range of +1 to +5. We will look at some of the important oxides below.
1) Dinitrogen Oxide, N2O
This is a colourless and non-flammable gaseous compound that has neutral properties. We know it by the common name, laughing gas. We can prepare it by the decomposing ammonium nitrate under high temperature.
NH4NO3 → N2O + 2H2O
2) Nitrogen Monoxide, NO
This is a colourless and gaseous compound. We can usually prepare it by reducing dilute nitric acid with copper.
2NaNO2 + 2FeSO4 + 3H2SO4 → Fe2(SO4)3 + 2NaHSO4 + 2H2O + 2NO
3) Dinitrogen Trioxide, N2O3
Dinitrogen trioxide is a deep blue solid. It has acidic properties. It is prepared by mixing equal parts of nitric oxide and nitrogen dioxide and by further cooling the mixture below −21 °C (−6 °F).
NO + NO2 → N2O3
4) Nitrogen Dioxide, NO2
Nitrogen dioxide is a common oxide of nitrogen. It is a reddish-brown toxic gas. We can know its presence with a sharp odour.
5) Dinitrogen Tetroxide, N2O4
Dinitrogen tetroxide is a colourless solid that we can find in equilibrium with nitrogen dioxide. It is a powerful oxidizer and is a common reagent in the production of many chemical compounds.
N2O4 ⇌ 2NO2
6) Dinitrogen Pentoxide, N2O5
Dinitrogen pentoxide is a colourless solid. Its characteristic property is that it sublimes slightly above room temperature. It is unstable. It is a potentially dangerous oxidizer. We can prepare it by dehydrating nitric acid (HNO3) with phosphorus (V) oxide:
P4O10 + 12HNO3 → 4H3PO4 + 6N2O5
Solved Example for You
Q: Give some uses of Nitric acid.
Ans: The essential uses of nitric acid are as follows:
- It plays the role of an important ingredient in explosives such as trinitrotoluene (T.N.T.), gun cotton etc.
- It is also important in the manufacture of fertilizers.
- We use it in the manufacturing of dyes, scents, drugs etc.
- Also, it is important for the cleaning of precious metals like gold, silver, platinum etc.






Leave a Reply