You must have noticed that a lot of ointments contain sulphur. However, are you aware “which” sulphur it is talking about? Did you know that there are more than one type of sulphur, even if you don’t see it on the periodic table? They are the allotropic forms of sulphur. So, what are these allotropic forms exactly? Let us read about their types, properties and uses. However, before we begin, let’s take a quick recap of the properties of sulphur.
Suggested Videos
What is Sulphur?
Sulphur is a chemical element having atomic number 16. It is easily accessible at room temperature. It is basically a splendid yellow crystalline solid. Sulphur is a non-metal, as we obviously know! The position of Sulphur in the periodic table is as follows:

Properties of Sulphur
The following are the most common properties of Sulphur. We will look at its chemical and physical properties separately.
Physical Properties
- Sulphur is yellow in colour.
- It is insoluble in water. However, it is very soluble in toluene (methylbenzene) and carbon disulphide.
- It is a non-metal and therefore, a poor conductor of electricity and heat.
- At the point, when we consolidate Sulphur vapour, we get a fine powder, which shapes a pattern resembling a flower. This is the ‘Flower of Sulphur’.
Browse more Topics under The P Block Elements
- Introduction to p Block Elements
- Some Important Compounds of Carbon and Silicon
- Trend and Anomalous Properties of Carbon
- Trends and Properties of Boron and Aluminium
- Ammonia
- Chlorine
- Dinitrogen
- Dioxygen
- Group 13 Elements: Boron Family
- Group 14 Elements: Carbon Family
- Group 15 Elements
- Group 16 Elements
- Group 17 Elements
- Group 18 Elements
- Hydrogen Chloride
- Interhalogen Compounds
- Nitric Acid and Oxides of Nitrogen
- Oxoacids of Halogens
- Oxoacids of Phosphorus
- Oxoacids of Sulphur
- Ozone
- Phosphine
- Phosphorus – Allotropic Forms
- Phosphorus Halides
- Simple Oxides
- Sulphuric Acid
- Sulphuric Dioxide
Chemical Properties
- Most metals and non-metals react with Sulphur, under specific conditions.
- Sulphur burns in excess of air with a bright blue fire and forms Sulphur (IV) oxide and a little amount of Sulphur (VI) oxide.
- It reacts with Hydrogen at high temperature and forms hydrogen sulphide.
- Sulphur vapour reacts with hot coke to produce a fluid, carbon disulphide.
Allotropic Forms of Sulphur
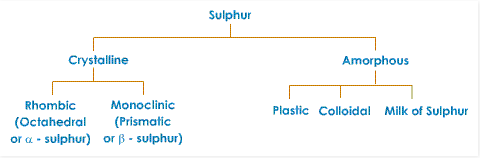
We can find sulphur in a number of structures in the same physical state. However, the most important crystalline structures are rhombic or octahedral (α – sulphur), and monoclinic sulfur (β – sulphur). We find the shape of rhombic sulphur at a temperature beneath 96oC. On the other hand, monoclinic sulphur occurs at a temperature over 96oC.
This temperature of 96oC is the transitional temperature between the two crystalline structures. There is another allotrope of sulphur, polymeric sulfur (S8). It is an eight-part ring particle. This is insoluble in organic media, synthetic and natural rubber. It also does not dissolve in carbon disulphide.
Let us now look at the properties of the two main allotropes of sulphur: rhombic and monoclinic sulphur.
1) Rhombic Sulphur
- We find them as yellow and translucent crystals.
- Rhombic Sulphur has a melting point of 114o C.
- The density of rhombic Sulphur is 2.08 g/cm3
- It is stable at temperatures below 96oC.
2) Monoclinic Sulphur
- These are transparent and amber crystals.
- They have a melting point of 119oC.
- The density of monoclinic sulphur is 1.98 gcm3
- It is unstable at temperatures below 96oC and changes into rhombic form.
- We must remember that at a temperature of 96oC or above, rhombic sulphur changes to kaleidoscopic or prismatic sulphur. At 96oC or beneath, kaleidoscopic or prismatic sulphur changes to rhombic sulphur.
- These allotropes that alter their configuration from one form to another by a change in the temperature are Enantiotropic Allotropes.
3) Colloidal Sulphur
- We can produce this sulphur by passing hydrogen sulphide through a saturated and cooled solution of sulphur dioxide in water. Another method is by including a solution of alcohol and sulphur in the water.
- It acts as a solvent in carbon disulfide.
- We utilise it as a part of medicines.
4) Milk of Sulphur
- We can produce this by the action of weak hydrochloric acid on ammonium sulphide. In a similar fashion, this milk of sulphur forms by the boiling of sulphur with calcium hydroxide (aqueous solution). We filter this mixture and add weak hydrochloric acid to get the milk of sulphur.
- This compound is non-crystalline and white in colour.
- It is soluble in carbon disulphide.
- At the point when we heat it, it changes to the conventional yellow colour of sulphur that we use as a part of medicines.
Solved Example for You
Q: Give some important uses of sulphur.
Ans: The most common uses of sulphur are:
- We use sulphur to develop specific sorts of fungus in vines.
- Sulphur is a common ingredient in the production of tetraoxosulphate(VI) acid. We can say, this is the most important use of sulphur.
- We use sulphur in the making of calcium hydrogen trioxosulphate(IV), Ca(HSO3)2. Here, this compound finds its use as a bleacher of wood pulp in the paper manufacturing industry.
- Sulphur is a common and important ingredient in the vulcanization of rubber. This process involves making the rubber tough and hard by binding the rubber molecules close to each other.
- We use sulphur in dye manufacturing.
- Sulphur is common in the fabrication of sulphur compounds, for example, carbon disulfide, CS2 and sulfur monochloride, S2Cl2.
- It finds its significant use in ointments.
- Sulphur is an important ingredient in sulphides like phosphorus sulphide. We use this as a part of making firecrackers, gunpowder and matches.






Leave a Reply